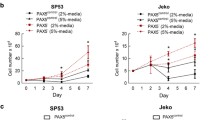
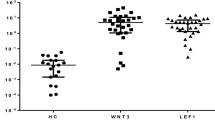
Abstract
Chronic lymphocytic leukemia (CLL) is a subtype of B-cell malignancy with high heterogeneity. XPO1 is highly expressed in many hematological malignancies, which predicts poor prognosis. In the study, we aimed to explore the prognostic role of XPO1 and the therapeutic effect of Selinexor, a selective inhibitor of nuclear export, which targets XPO1. We collected 200 CLL samples in our center to confirm XPO1 mRNA expression and analyzed the correlation between XPO1 expression and prognosis. Then, we decreased XPO1 expression with Selinexor to explore the effect of proliferation inhibition, cell cycle arrest, and apoptosis in CLL cell lines. RNA-Seq was performed to explore potential mechanisms. We analyzed XPO1 expression in a cohort of 150 treatment naive patients and another cohort of 50 relapsed and refractory (R/R) patients and found that XPO1 expression was upregulated in 76% of CLL patients compared with healthy donors. Survival analysis suggested that patients with increased XPO1 expression had inferior treatment-free survival (P = 0.022) and overall survival (P = 0.032). The inhibitor of XPO1, Selinexor, induced apoptosis in primary CLL cells. We showed the effects of Selinexor on proliferation inhibition, cell cycle arrest, and apoptosis in CLL cell lines with JVM3, MEC1, and ibrutinib-resistant (MR) cells via nuclear retention of cargo proteins of IκBα, p65, p50, and FOXO3a. Moreover, downregulation of the NF-κB and FOXO pathways was a common feature of the three CLL cell lines responding to Selinexor, indicating the potential application of XPO1 inhibitor even in the high-risk CLL cells. We identified XPO1 as an unfavorable prognostic factor for CLL patients and provided a rationale for further investigation of the clinically XPO1 targeted therapeutic strategy against CLL.






Similar content being viewed by others
Availability of data and material
The authors declare that all data and materials are available on request.
References
Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet. 2018;391(10129):1524–37.
Scarfo L, Ferreri AJ, Ghia P. Chronic lymphocytic leukaemia. Crit Rev Oncol Hematol. 2016;104:169–82.
Sharma S, Rai KR. Chronic lymphocytic leukemia (CLL) treatment: So many choices, such great options. Cancer. 2019;125(9):1432–40.
Albiol N, Arguello-Tomas M, Moreno C. The road to chemotherapy-free treatment in chronic lymphocytic leukaemia. Curr Opin Oncol. 2021;33(6):670–80.
Hutten S, Kehlenbach RH. CRM1-mediated nuclear export: to the pore and beyond. Trends Cell Biol. 2007;17(4):193–201.
Azmi AS, Uddin MH, Mohammad RM. The nuclear export protein XPO1—from biology to targeted therapy. Nat Rev Clin Oncol. 2021;18(3):152–69.
Kojima K, Kornblau SM, Ruvolo V, et al. Prognostic impact and targeting of CRM1 in acute myeloid leukemia. Blood. 2013;121(20):4166–74.
Ming M, Wu W, Xie B, et al. XPO1 inhibitor Selinexor overcomes intrinsic Ibrutinib resistance in mantle cell lymphoma via nuclear retention of IkappaB. Mol Cancer Ther. 2018;17(12):2564–74.
Zhang K, Wang M, Tamayo AT, et al. Novel selective inhibitors of nuclear export CRM1 antagonists for therapy in mantle cell lymphoma. Exp Hematol. 2013;41(1):67-78e64.
Lapalombella R, Sun Q, Williams K, et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood. 2012;120(23):4621–34.
Liscic RM, Alberici A, Cairns NJ, Romano M, Buratti E. From basic research to the clinic: innovative therapies for ALS and FTD in the pipeline. Mol Neurodegener. 2020;15(1):31.
Ranganathan P, Yu X, Na C, et al. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood. 2012;120(9):1765–73.
Benkova K, Mihalyova J, Hajek R, Jelinek T. Selinexor, selective inhibitor of nuclear export: Unselective bullet for blood cancers. Blood Rev. 2021;46: 100758.
Syed YY. Selinexor: first global approval. Drugs. 2019;79(13):1485–94.
Kasamon YL, Price LSL, Okusanya OO, et al. FDA approval summary: selinexor for relapsed or refractory diffuse large B-cell lymphoma. Oncologist. 2021;26(10):879–86.
Hing ZA, Mantel R, Beckwith KA, et al. Selinexor is effective in acquired resistance to ibrutinib and synergizes with ibrutinib in chronic lymphocytic leukemia. Blood. 2015;125(20):3128–32.
Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–60.
Melo JV, Brito-Babapulle V, Foroni L, Robinson DS, Luzzatto L, Catovsky D. Two new cell lines from B-prolymphocytic leukaemia: characterization by morphology, immunological markers, karyotype and Ig gene rearrangement. Int J Cancer. 1986;38(4):531–8.
Stacchini A, Aragno M, Vallario A, et al. MEC1 and MEC2: two new cell lines derived from B-chronic lymphocytic leukaemia in prolymphocytoid transformation. Leuk Res. 1999;23(2):127–36.
Zhao X, Lwin T, Silva A, et al. Unification of de novo and acquired ibrutinib resistance in mantle cell lymphoma. Nat Commun. 2017;8:14920.
Deng M, Zhang M, Xu-Monette ZY, et al. XPO1 expression worsens the prognosis of unfavorable DLBCL that can be effectively targeted by selinexor in the absence of mutant p53. J Hematol Oncol. 2020;13(1):148.
Aladhraei M, Kassem Al-Thobhani A, Poungvarin N, Suwannalert P. Association of XPO1 overexpression with NF-kappaB and Ki67 in colorectal cancer. Asian Pac J Cancer Prev. 2019;20(12):3747–54.
Saulino DM, Younes PS, Bailey JM, Younes M. CRM1/XPO1 expression in pancreatic adenocarcinoma correlates with survivin expression and the proliferative activity. Oncotarget. 2018;9(30):21289–95.
Jain P, Kanagal-Shamanna R, Wierda W, et al. Clinical and molecular characteristics of XPO1 mutations in patients with chronic lymphocytic leukemia. Am J Hematol. 2016;91(11):E478–9.
Yoshimura M, Ishizawa J, Ruvolo V, et al. Induction of p53-mediated transcription and apoptosis by exportin-1 (XPO1) inhibition in mantle cell lymphoma. Cancer Sci. 2014;105(7):795–801.
Nie D, Xiao X, Chen J, et al. Prognostic and therapeutic significance of XPO1 in T-cell lymphoma. Exp Cell Res. 2022;416(2): 113180.
Wang J, Sun T, Meng Z, et al. XPO1 inhibition synergizes with PARP1 inhibition in small cell lung cancer by targeting nuclear transport of FOXO3a. Cancer Lett. 2021;503:197–212.
Kim J, McMillan E, Kim HS, et al. XPO1-dependent nuclear export is a druggable vulnerability in KRAS-mutant lung cancer. Nature. 2016;538(7623):114–7.
Azizian NG, Li Y. XPO1-dependent nuclear export as a target for cancer therapy. J Hematol Oncol. 2020;13(1):61.
Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–74.
Imbert V, Peyron JF. NF-kappaB in hematological malignancies. Biomedicines. 2017;5:2.
Ecker V, Stumpf M, Brandmeier L, et al. Targeted PI3K/AKT-hyperactivation induces cell death in chronic lymphocytic leukemia. Nat Commun. 2021;12(1):3526.
Bertacchini J, Heidari N, Mediani L, et al. Targeting PI3K/AKT/mTOR network for treatment of leukemia. Cell Mol Life Sci. 2015;72(12):2337–47.
Nair JS, Musi E, Schwartz GK. Selinexor (KPT-330) induces tumor suppression through nuclear sequestration of IkappaB and downregulation of survivin. Clin Cancer Res. 2017;23(15):4301–11.
DeSisto JA, Flannery P, Lemma R, et al. Exportin 1 inhibition induces nerve growth factor receptor expression to inhibit the NF-kappaB Pathway in preclinical models of pediatric high-grade glioma. Mol Cancer Ther. 2020;19(2):540–51.
Kashyap T, Argueta C, Aboukameel A, et al. Selinexor, a selective inhibitor of nuclear export (SINE) compound, acts through NF-kappaB deactivation and combines with proteasome inhibitors to synergistically induce tumor cell death. Oncotarget. 2016;7(48):78883–95.
Link W. Introduction to FOXO biology. Methods Mol Biol. 2019;1890:1–9.
Kapoor I, Li Y, Sharma A, et al. Resistance to BTK inhibition by ibrutinib can be overcome by preventing FOXO3a nuclear export and PI3K/AKT activation in B-cell lymphoid malignancies. Cell Death Dis. 2019;10(12):924.
Stephens DM, Huang Y, Ruppert AS, et al. Selinexor combined with ibrutinib demonstrates tolerability and safety in advanced B-cell malignancies: a phase I study. Clin Cancer Res. 2022;28(15):3242–7.
Luedtke DA, Su Y, Liu S, et al. Inhibition of XPO1 enhances cell death induced by ABT-199 in acute myeloid leukaemia via Mcl-1. J Cell Mol Med. 2018;22(12):6099–111.
Yu H, Wu S, Liu S, et al. Venetoclax enhances DNA damage induced by XPO1 inhibitors: a novel mechanism underlying the synergistic antileukaemic effect in acute myeloid leukaemia. J Cell Mol Med. 2022;26(9):2646–57.
Acknowledgements
We would like to acknowledge the patients who volunteered to participate in this study and the Core Facility of the First Affiliated Hospital of Nanjing Medical University for their instructions.
Funding
This work was supported by National Natural Science Foundation of China (81800192, 82100207, 82200210, 82100207), Nature Science Foundation for Youths of Jiangsu Province (BK20210962, BK2021041892, BK2022043464, BK20210962), and Young Scholars Fostering Fund of the First Affiliated Hospital of Nanjing Medical University (PY2021026).
Author information
Authors and Affiliations
Contributions
ZX carried out most of the experiments. BP and JW provided the study design. ZX and BP participated in writing the article and making figures and tables. YL and YX were responsible for analyzing data and interpreting results. Sample collection and clinical data interpretation were completed by YM, SQ, JL, XZ, JT, HZ and LW. JL, JW, and WX provided instructions and revised the paper. Final approval of the manuscript was performed by all authors who contributed to the article.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval and consent to participate
The study was approved by the First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital. And written informed consent was obtained from all patients according to the Declaration of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.



Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Z., Pan, B., Miao, Y. et al. Prognostic value and therapeutic targeting of XPO1 in chronic lymphocytic leukemia. Clin Exp Med 23, 2651–2662 (2023). https://doi.org/10.1007/s10238-023-01003-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01003-6