Abstract
Acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) are malignant clonal diseases of the hematopoietic system with an unsatisfactory overall prognosis. The main obstacle is the increased resistance of AML and ALL cells to chemotherapy. The development and validation of new therapeutic strategies for acute leukemia require preclinical models that accurately recapitulate the genetic, pathological, and clinical features of acute leukemia. A patient-derived orthotopic xenograft (PDOX) model is established using surgical orthotopic implantation. They closely resemble human tumor progression and microenvironment and are more reliable translational research tools than subcutaneous-transplant models. In this study, we established PDOX models by direct intrafemoral injection of bone marrow and peripheral blood cells from AML and ALL patients, characterized their pathology, cytology, and genetics, and compared the model's characteristics and drug responsiveness with those of the corresponding patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leukemia is a common malignancy worldwide. Acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) are monoclonal neoplastic proliferations of myeloid precursors or lymphoid progenitor cells. Ongoing advances in treatment have significantly improved 5-year survival, but the overall prognosis of acute leukemia is not satisfactory [1]. About 50% of AML patients and 20% of ALL patients experience a relapse, which is the greatest challenge in the management of acute leukemia [2, 3]. The main obstacle in the treatment of acute leukemia is the increased resistance of AML and ALL cells to chemotherapy [4, 5]. The development and validation of novel therapeutic strategies for acute leukemia require preclinical models that accurately recapitulate the genetic, pathological, and clinical characteristics of AML and ALL.
Preclinical experimental models of acute leukemia are limited because they differ from human disease in genetic heterogeneity, pathological characteristics, and drug responsiveness [6]. Patient-derived xenograft (PDX) models are established by transplantation of patient tumor tissue or cells into immunocompromised mice, the strains of which were developed for PDXs including nude (nu), severe combined immunodeficient (SCID), non-obese diabetic (NOD), NOD-SCID, and NOD-SCID-IL2rγnull (NSG) strains [7, 8]. In one of the first acute leukemia PDX studies, researchers transplanted AML cells into nude mice; however, due to an intact B cell and NK cell function, grafting of acute leukemic cells in nude mice remained poor. Then the development of acute leukemia mouse models in severe combine immunodeficient (SCID) mice was an important step forward. SCID mice are lack of functional mature T and B cells, but retain NK function. Thus, primary AML injected intraperitoneally or implanted under the kidney capsules showed improved engraftment rates in SCID mice, but intravenous injection still remained poor and unreliable. To overcome these limitations above, researchers began to transfer patient-derived leukemic cells directly into BM by intrafemoral injection, which was regarded as one kind of the patient-derived orthotopic xenograft (PDOX) models with implantation of tumor cells into the corresponding anatomical position in mice [9, 10]. Actually since 1990s of the last century, PDOX models have been developed using samples obtained at surgery for patients with solid tumors including colon cancer, pancreatic cancer, gastric cancer, breast cancer, lung cancer and many other cancers [11]. To further improve engraftment rates, mouse models with more severe immunodeficiency strains such as NSG mice were developed by combining the SCID background with the non-obese diabetic (NOD) strain which have no functional B or T cells and reduced NK cell and macrophage activity. They showed superior engraftment rate compared to SCID mice even when injecting fewer primary leukemic cells. Moreover, the morphologic, phenotypic, and genetic characteristics of the expanded AML specimens seemed mostly preserved [10].
This study investigated the usefulness of PDOX mouse models of AML and ALL for the study of drug sensitivity in NCG mice. NCG mice as a kind of more severe immune-deficient strain being fairly similar to NSG mice was established by CRISPR/Cas9 technology. Prkdc (Protein kinase, DNA activated, catalytic polypeptide) and Il2rg (Common gamma chain receptor) genes were knocked out on NOD/ShiltJGpt background. The genetic background of NOD/ShiltJGpt resulted in natural immunodeficiency, such as complement system and macrophage defects [12]. Meanwhile, the Sirpa on NOD/ShiltJGpt has high affinity with human CD47, making it more suitable for colonization of human grafts (e.g., tumors and human cells) than other strains [13]. Loss of Prkdc gene leads to the inability of V(D)J recombination to occur, resulting in the inability of T cells and B cells to mature. Il2rg is a common subunit of various interleukin cytokine receptors, and the inactivation of Il2rg leads to the loss of six different cytokine signaling pathways, resulting in NK cell defects [14]. We established models from patients with AML and ALL, characterized their pathological, cytological, and genetic features, and compared the features and drug responsiveness of the models with those of the corresponding patients.
Materials and methods
AML and ALL patient samples
Peripheral blood or bone marrow samples were obtained from AML and ALL patients at the China–Japan Union Hospital of Jilin University. The study was reviewed and approved by the Institutional Ethics Committee, and written informed consent for study enrollment and blood or bone marrow collections was obtained from all patients.
Antibodies and other reagents
Antibodies (Abs) used for flow cytometry, PE mouse antihuman CD33 (Cat#: 555,450) and APC mouse antihuman CD45 (Cat#: 555,485) were purchased from BD Biosciences, PerCP/Cy5.5 antihuman CD19 (Cat#: 302,229) was purchased from Biolegend. Cytarabine (Cat#: BD8499), imatinib (Cat#: BD42606), and ibrutinib (Cat#:BD254580) were purchased from Bide Pharmatech (Shanghai, China). Epirubicin (Cat#: 56,390-09-1) was purchased from Shandong New Time Pharmaceutical Co., Ltd., Company (Shandong, China). Vincristine (Cat#: MB1298) was purchased from Melone Pharma (Dalian, China). Cladribine (Cat#:CSN10004) was purchased from CSNpharma (Shanghai, China).
Animals
The immunodeficient NCG (NOD/ShiLtJ-Prkdc em26Cd52 Il2rg em26Cd22) mice used in this study were purchased from GemPharmatech Co., Ltd. Company (Nanjing, China) and maintained in pathogen-free conditions at Shanghai LIDE Biotechnology Co., Ltd., Company (Shanghai, China).
Establishment of the PDOX models using peripheral blood or bone marrow
6- to 8-week-old NCG mice were injected intrafemorally with 1–2 × 106 peripheral blood or bone marrow cells of AML or ALL patients after being anesthetized with a 1.25% avertin solution, respectively.
Evaluation of the PDOX models and patient samples
Flow cytometry
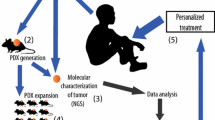
Engraftment of the orthotopic models was monitored by flow cytometry of peripheral blood from inoculated mice. The frequencies of CD45+CD33+ expression in AML and CD45+CD19+ expression for B-ALL were assayed. A 5–15% dual positivity indicated AML or ALL PDOX engraftment. The procedures of PDOX establishment are shown in Fig. 1. Staining and flow cytometry were performed following the manufacturers’ protocols. An Attune NxT flow cytometer was used with Attune NxT v4.2.0 software.
Additional analysis
The presence of gene mutations that commonly occur in AML and ALL patients was investigated by next generation sequencing of peripheral blood and bone marrow samples obtained from the AML and B-ALL patients and the xenografts in the PDOX mouse spleen samples. Chromosomal analysis of human AML and ALL cells in mouse spleen samples from the established PDOX models was performed by karyotyping and standard G-banding.
Standard of care in AML and ALL PDOX models
Cladribine, cytarabine, imtatinib, or epirubicin in combination with vincristine were used to validate the in vivo efficacy of standard chemotherapies in the AML and ALL PDOX models. Cladribine and imatinib was given daily via gavage at 0.4 mg/dose and 25 mg/kg, respectively. Cytarabine was given on days 1–5 by intraperitoneal injection at 10 mg/kg. Epirubicin was given weekly by intraperitoneal injection at 1 or 2.5 mg/kg. Vincristine was given weekly by intravenous injection at 0.5 mg/kg. The body weight of each animal was recorded every day, and doses were normalized against the individual weight to ensure consistency among groups. Flow cytometry was performed weekly to determine AML/ALL progression and the anti-tumor effectiveness of each treatment.
Results
Identification of patient samples
In the validation phase, we identified potential markers and targets in patient samples by next generation sequencing, G-banding, and flow cytometry, and the results are shown in Table 1.
Engraftment of AML and ALL PDOX model evaluated by fluorescence-activated cell sorting (FACS)
Patient characteristics, diagnosis, and treatment history, are shown in Table 2. During this study, 67 samples of the peripheral blood (PB) or bone marrow (BM) of AML patients and 20 samples of the peripheral blood (PB) or bone marrow (BM) of ALL patients were processed for PDOX model establishment, which were prepared by intrafemoral injection of fresh blasts from the peripheral blood (PB) or bone marrow (BM) of AML and ALL patients into NCG mice and screened for their potential to initiate leukemia in the mouse models. The frequency of CD45+CD33+ cells or CD45+ cells in mouse peripheral blood was monitored weekly by FACS beginning 3–4 weeks after transplantation. To confirm the engraftment of human AML/ALL in the mouse models, P0 indicates the primary passage of patient cell suspension prepared from patient blood or bone marrow samples. When the percentage of positive cells reached 10%, single cell suspensions prepared from the mouse spleen were inoculated intrafemorally into naïve NCG mice (P1), or cryopreserved in liquid nitrogen until inoculation (FP0 + 1). The percentages of leukemia cells at P0, P1, and FP0 + 1 in 12 representative PDOX models are shown in Fig. 2. Successfully engraftment of PDOX models was defined as 10% CD45+CD33+ or CD45+ at P1. Successfully established PDOX required stable morphologic and molecular characteristics for at least two passages. In terms of our success tumor take rates, 10.45% (7/67) of AML patient samples and 40%(8/20) of ALL patient samples successfully initiated PDOX models. In addition, our study indicated that leukemia developed initially from the bone marrow of mice. After 5 weeks of transplantation in LD1-0024-361,280 AML PDOX model, the samples of the peripheral blood (PB) or bone marrow (BM) in 6 mice were collected at the same time for FACS detection. The results showed that the mean percentage of CD45+ CD33+ cells in the peripheral blood (PB) was 13.80% and the mean percentage of CD45+ CD33+ cells in bone marrow was 62.02%.
Establishment of PDOX Model in NCG mice. Cell suspensions prepared from patient samples were injected intrafemorally into NCG mice to generate the PDOX models. The frequency and percentage of CD45 + or CD45 + CD33 + cells in mouse peripheral blood were determined by FACS at P0 (black lines), P1 (red lines), and FP0 + 1 (blue lines) of established PDOX models. Individual mice are shown as point values. Group results are shown with bars that give the standard deviation
Cell suspensions prepared from patient samples were injected intrafemorally into NCG mice to generate the PDOX models. The frequency and percentage of CD45 + or CD45 + CD33 + cells in mouse peripheral blood were determined by FACS at P0 (black lines), P1 (red lines), and FP0 + 1 (blue lines) of established PDOX models. Individual mice are shown as point values. Group results are shown with bars that give the standard deviation.
Consistency of cell surface markers in patient samples and PDOX models
FACS analysis of cell surface markers in peripheral blood of patient samples and the PDOX models established using the samples are shown in Table 3. CD19 was positive in both patient samples and all four of the corresponding PDOX ALL models. The ALL were B cell-derived phenotypes. All four were CD10+ and CD38+, suggesting that CD19+CD10+CD38+ cell populations might be a useful as a panel to determine the establishment of PDOX in this type of B-ALL. The series of ALL-associated antigens revealed that the immunophenotype of PDOX cells was consistent with the primary specimens for most antigens tested and that they preserved the disease characteristics of the patients that they originated from.
Chromosome analysis of clinical samples and PDOX cells
G-banding karyotype analysis showed normal karyotypes and revealed that the karyotypes of PDX cells of LD1-0041-362,073 and LD1-0041-362,021 were similar to that of patient specimens. However, LD1-1041-362,519 showed a different karyotype in the PDX versus patient, the discordance between cytogenetic result of clinical specimen and PDOX on 1q21 and 18q21 may result from clonal selection. Moreover, because there is a complex structural variation on chromosome #9, it is uncertain whether it is a discordance (may also result from clonal selection) or different resolution, it would be better to have more clinical data to verify this. (Table 4, Fig. 3).
Karyotypes A of peripheral blood from an ALL patient (model ID: LD1-0041-362,073) and of a mouse peripheral blood from the LD1-0041-362,073 PDOX model. B Karyotypes of bone marrow from an ALL patient (model ID: LD1-1041-362,519) and of b mouse bone marrow from the LD1-1041-362,519 PDOX model. C Karyotypes of bone marrow of an ALL patient (model ID: LD1-0041-362,021) and c mouse bone marrow of the LD1-0041-362,021 PDOX model
PDOX and the parental clinical sample have similar genetic profiles
An AML patient (LD1-0040-361,280) had a mutation on exon 12 of NPM1, a typical hot-spot alteration seen in AML patients without chromosome abnormalities [13]. As shown in Table 5, an NPM1exon12A mutation was found in the PDOX model by either RNAseq or whole exon sequencing. Other mutations such as c-kit/D816V, CEBPA, or FLT3/ITD that are often found in AML were not present in either the PDOX model or the original patient sample. The result indicates consistency in the genetic profiles of the patient and the PDOX model.
Standard of care validation with therapeutic regimens used in clinical practice
Validation using the same treatments that were administered to patient was performed in five of the established PDOXs. Cytarabine, which is commonly given to AML/ALL, patents had anti-tumor activity in both the AML and the ALL PDOXs (Fig. 4B–E). In the AML PDOX, cladribine completely eliminated the tumor (Fig. 4A). Epirubicin and vincristine, which are components in CEOP treatment [14] had good anti-tumor activity in three of the four ALL PDOX models (Fig. 4C–E), but not in the LD1-0040-362,519 model (Fig. 4F). Imatinib and ibrutinib, two small molecules that target BCR-Abl and BTK, respectively, were not effective in all the PDOX models (Fig. 4C and F). As shown in Table 2, response or non-response of the PDOX models was consistent with the clinical outcomes that occurred in response to those agents by the corresponding patients. The patient responses to treatment were maintained in the PDOX mouse models.
Standard of care validation in PDOX models. AML PDOX (LD1-0040-361,280) and ALL PDOXs (LD1-0040-362,073, LD1-0040-362,356, LD1-0040-362,021, and LD1-0040-362,519) models were used for standard of care validation. Treatment of the model mice began after randomization to the indicated regimen. Cell surface markers (CD45+, CD33+, and/or CD19+) were monitored weekly until the end of the study. Data are means ± standard deviation and p < 0.05 indicates statistical significance versus control
AML PDOX (LD1-0040-361,280) and ALL PDOXs (LD1-0040-362,073, LD1-0040-362,356, LD1-0040-362,021, and LD1-0040-362,519) models were used for standard of care validation. Treatment of the model mice began after randomization to the indicated regimen. Cell surface markers (CD45+, CD33+, and/or CD19+) were monitored weekly until the end of the study. Data are means ± standard deviation and p < 0.05 indicates statistical significance vs. control.
Discussion
Patient-derived xenografts(PDX) are based on transferring primary tumors directly from the patient into an immunodeficient mouse. There are several advantages with PDXs including retaining the histological characteristics of patient tumors, such as tumor heterogeneity and tumor microenvironment [15]. When compared with traditional subcutaneous-transplant PDX model, PDOX models allowed the evaluation of tumor behavior in an organ-specific microenvironment. Furthermore, the PDOX models have been shown to have advantages over subcutaneous-transplant models, particularly with metastasis [11, 16]. AML and ALL PDOX mouse models were established by intrafemoral injection of leukemia cells from patients into triple immunodeficient NCG mice. The xenografted mouse models faithfully mimicked the pathology, cytology, karyotype, and genetic profile of the corresponding patient samples. The responsiveness of the AML and ALL PDOX mouse models to chemotherapy drugs was highly consistent with the clinical outcomes of the corresponding patients. The findings suggest that the AML and ALL PDOX mouse models established in this study preserved the features of the disease in the patients.
Heterogeneity of the malignant cells, with multiple mutant clones and subclones is a hallmark of acute leukemia [17, 18]. Tumor cell heterogeneity in acute leukemia reduces the effectiveness of treatment [19]. Culturing and passaging exert a selective pressure on leukemia cells that favors the survival of undifferentiated cells and results in loss of original tumor cell heterogeneity [20]. Therefore, the behavior of leukemia cell lines is profoundly different from the patient’s original tumor. PDX models of AML and ALL, which better represent the genetic and phenotypic heterogeneity of the corresponding leukemia, are indispensable for translational studies in acute leukemia [21, 22]. Establishment of PDX models of hematological malignancies remains challenging because leukemia cells have low take rate when transplanted into mice. The low success rate might be the result residual natural killer (NK) cell activity in non-obese diabetic (NOD)/severe combined immunodeficient (SCID) mice [23,24,25]. Crossbreeding NOD/SCID and interleukin (IL)-2 receptor γ-deficient mice has produced triple immunodeficient NCG, NSG, or NOG mice that lack functional T, B, and NK cells and are available from a number of suppliers [26]. The use of the triple immunodeficient NSG and NOG mouse models has significantly improved the efficiency of ALL and AML tumor engraftment in mouse models [27,28,29]. Triple immunodeficient NCG mice are not yet widely used to generate PDX models. This study demonstrated that NCG mice are also a kind of ideal models for the establishment of AML and ALL PDX models.
The implantation site of cancer cells affects the growth characteristics and phenotype of the PDX model [30]. Early PDX models of leukemia were established by heterotopically implanting cancer cells into the subcutaneous or intravenous space [9, 17, 23], and patient-derived heterotopic xenografts are widely used in cancer research. In this study, leukemia cells were injected intrafemorally into NCG mice to generate PDOXs. It is technically easier to perform and monitor tumor size of heterotopic implantation than establishing PDOXs [21]. Establishment of the leukemia PDOX model is time-consuming and technically challenging. The main advantage of PDOX models is a preserved tumor microenvironment and better modeling of tumor metastasis than heterotopic implantation [18].
An ideal research model should recapitulate the phenotypic and genetic characteristics of the original tumor in patients. In this study, the pathology of the mouse spleen and the surface markers of the engrafted cells from the PDOX models faithfully resembled the phenotypes of the patient leukemia samples. The karyotypes of PDOX cells were also highly consistent with those of the patient samples. We also compared the genetic profiles of PDOX models, and the clinical samples used for grafting by next generation sequencing. The NPM1 gene is the most frequently mutated gene in AML. We found that the NPM1 gene mutation in the PDOX model was consistent with the patient’s bone marrow sample, suggesting the PDOX model has stable genetic profiling.
In conclusion, it is feasible to establish AML and ALL PDOX models using the triple immunodeficient NCG mouse model. The established PDOX models preserve the pathological, phenotypic, and genetic characteristics of the clinical samples, and they also have similar drug sensitivity to the corresponding patients. The PDOX mouse model of acute leukemia provides a clinically relevant platform for testing novel chemotherapy drugs. Ultimately, the PDOX mouse model may be used for developing precision medicine approaches to treat leukemia.
References
Kantarjian HM, et al. Acute myeloid leukemia: treatment and research outlook for 2021 and the MD Anderson approach. Cancer. 2021;127(8):1186–207.
Thol F, Ganser A. Treatment of relapsed acute myeloid leukemia. Curr Treat Options Oncol. 2020;21(8):66.
Samra B, et al. Evolving therapy of adult acute lymphoblastic leukemia: state-of-the-art treatment and future directions. J Hematol Oncol. 2020;13(1):70.
Zhang J, Gu Y, Chen B. Mechanisms of drug resistance in acute myeloid leukemia. Onco Targets Ther. 2019;12:1937–45.
Kantarjian H, et al. Acute myeloid leukemia: current progress and future directions. Blood Cancer J. 2021;11(2):41.
Sajjad H, et al. Cancer models in preclinical research: a chronicle review of advancement in effective cancer research. Animal Model Exp Med. 2021;4(2):87–103.
Jin K, et al. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin Transl Oncol. 2010;12(7):473–80.
Sia D, et al. The future of patient-derived tumor xenografts in cancer treatment. Pharmacogenomics. 2015;16(14):1671–83.
Hoffman RM. Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer. 2015;15(8):451–2.
Almosailleakh M, Schwaller J. Murine models of acute myeloid leukaemia. Int J Mol Sci. 2019;20(2):635.
Cornett A, et al. Serial patient-derived orthotopic xenografting of adenoid cystic carcinomas recapitulates stable expression of phenotypic alterations and innervation. EBioMedicine. 2019;41:175–84.
Lee S, Kivimae S, Szoka FC. Clodronate improves survival of transplanted hoxb8 myeloid progenitors with constitutively active GMCSFR in immunocompetent mice. Mol Ther Methods Clin Dev. 2017;7:60–73.
Wen H, et al. Recurrent ECSIT mutation encoding V140A triggers hyperinflammation and promotes hemophagocytic syndrome in extranodal NK/T cell lymphoma. Nat Med. 2018;24(2):154–64.
Cao Z, et al. Targeting the vascular and perivascular niches as a regenerative therapy for lung and liver fibrosis. Sci Transl Med. 2017;9(405):10574.
Bisht S, Feldmann G. Animal models for modeling pancreatic cancer and novel drug discovery. Expert Opin Drug Discov. 2019;14(2):127–42.
Hoffman RM. Patient-derived orthotopic xenograft (PDOX) Models of melanoma. Int J Mol Sci. 2017;18(9):1875.
Horibata S, et al. Heterogeneity in refractory acute myeloid leukemia. Proc Natl Acad Sci U S A. 2019;116(21):10494–503.
Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501(7467):328–37.
Lai Y, et al. Current status and perspectives of patient-derived xenograft models in cancer research. J Hematol Oncol. 2017;10(1):106.
Belderbos ME, et al. Clonal selection and asymmetric distribution of human leukemia in murine xenografts revealed by cellular barcoding. Blood. 2017;129(24):3210–20.
Hidalgo M, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013.
Clappier E, et al. Clonal selection in xenografted human T cell acute lymphoblastic leukemia recapitulates gain of malignancy at relapse. J Exp Med. 2011;208(4):653–61.
Bhimani J, Ball K, Stebbing J. Patient-derived xenograft models-the future of personalised cancer treatment. Br J Cancer. 2020;122(5):601–2.
Okada S, Vaeteewoottacharn K, Kariya R. Application of highly immunocompromised mice for the establishment of patient-derived xenograft (PDX) models. Cells. 2019;8(8):889.
Lock RB, et al. The nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse model of childhood acute lymphoblastic leukemia reveals intrinsic differences in biologic characteristics at diagnosis and relapse. Blood. 2002;99(11):4100–8.
Lodhia KA, et al. Prioritizing therapeutic targets using patient-derived xenograft models. Biochim Biophys Acta. 2015;1855(2):223–34.
Hassan N, Yang J, Wang JY. An improved protocol for establishment of AML patient-derived xenograft models. STAR Protoc. 2020;1(3):100156.
Tomii T, et al. Leukemic cells expressing NCOR1-LYN are sensitive to dasatinib in vivo in a patient-derived xenograft mouse model. Leukemia. 2021;35(7):2092–6.
Loftus JP, et al. Combinatorial efficacy of entospletinib and chemotherapy in patient-derived xenograft models of infant acute lymphoblastic leukemia. Haematologica. 2021;106(4):1067–78.
Schueler J, et al. Impact of the injection site on growth characteristics, phenotype and sensitivity towards cytarabine of twenty acute leukaemia patient-derived xenograft models. Cancers (Basel). 2020;12(5):1349.
Acknowledgments
We are grateful to Yanbin Zhou, Zheng Yang, and Shuai Wang for their contributions to the success of the study.
Funding
This work was supported by Division of Social science and Technology Development, Science and Technology Department of Jilin Province, China (Project Number: 20210203067SF)
Author information
Authors and Affiliations
Contributions
LB and DW designed this study. JL, HC, and SZ, performed experiments. LB, DW, JL, HC, and SZ analyzed the data in this paper. JL and HC wrote the manuscript and prepared Figs. 1, 2, 3, 4 and Tables 1, 2, 3, 4, 5. LB and DW revised the manuscript. LB and DW reviewed and supervised the experiments. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Chen, H., Zhao, S. et al. Patient-derived intrafemoral orthotopic xenografts of peripheral blood or bone marrow from acute myeloid and acute lymphoblastic leukemia patients: clinical characterization, methodology, and validation. Clin Exp Med 23, 1293–1306 (2023). https://doi.org/10.1007/s10238-022-00884-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00884-3