Abstract
Purpose
Lugol’s solution could control thyroid function and suppress 131I uptake in hyperthyroidism. This study aimed to investigate the appropriate time to withdraw Lugol’s solution before 131I therapy (RIT) in Graves’ disease (GD) patients, and how this should influence 131I uptake and RIT outcome.
Methods
Two groups (125 cases and 1805 cases) of GD patients received RIT, who were pre-treated with and without Lugol’s solution (RI-CI group and RI group). The RI-CI group was further divided into the following sub-groups depending on the duration span between Lugol’s solution withdrawal and RIT: sub-group A, 4–7 d (n = 49); sub-group B, 8–14 d (n = 41); and sub-group C, 15–30 d (n = 35). The highest radioactive iodine uptake rate (RAIUmax), effective half-life (Teff), TRAb, and free triiodothyronine (FT3) and free thyroxine (FT4) levels were compared, and therapeutic outcome was evaluated.
Results
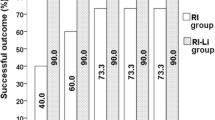
There were no significant differences in RAIUmax, TRAb, and Teff among the four sub-groups (P > 0.05). Both FT3 and FT4 levels in sub-groups A and B were lower than those in group RI and sub-group C (P < 0.05). The outcome of non-hyperthyroidism (euthyroidism + hypothyroidism) in groups RI-CI and RI was significantly different at post-RIT month 1 and 3 (P < 0.05). However, intergroup differences at 6 and 12 months were not significant (P > 0.05).
Conclusions
Withdrawal of Lugol’s solution 4–7 or 8–14 d before RIT does not influence 131I uptake and RIT efficacy in GD. Moreover, in order to avoid a rapid increase in thyroid hormone levels at the same time, Lugol’s solution should be withdrawn 4–7 d before RIT.

Similar content being viewed by others
Availability of data and materials
The data of this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
References
Bahn Chair RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593–646.
Jiang NY, Lin YS, Guan HX, et al. Clinical guidelines for 131I treatment of Graves hyperthyroidism. Chin J Nucl Med Mol Imaging. 2013;33:83–95.
Bonnema SJ, Hegedüs L. Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev. 2012;33:920–80.
Sfiligoj D, Gaberscek S, Mekjavic PJ, et al. Factors influencing the success of radioiodine therapy in patients with Graves’ disease. Nuclear Med Commun. 2015;36:560–5.
Willegaignon J, Sapienza MT, Coura-Filho GB, et al. Graves’ disease radioiodine-therapy: choosing target absorbed doses for therapy planning. Med Phys. 2014;41:012503.
Wang J, Qin L. Radioiodine therapy versus antithyroid drugs in Graves’ disease: a meta-analysis of randomized controlled trials. Br J Radiol. 2016;89:20160418.
Zheng W, Jian T, Guizhi Z, et al. Analysis of 131I therapy and correlation factors of Graves’ disease patients: a 4-year retrospective study. Nucl Med Commun. 2012;33:97–101.
Gierach M, Gierach J, Pilecki S, et al. [The estimation of the goiter by means of ultrasonography and scintigraphy (SPECT) with using 131I. Endokrynol Polska. 2007;58:403–7.
Marinelli LD, Quimby EH, Hine GJ. Dosage determination with radioactive isotopes; practical considerations in therapy and protection. Am J Roentgenol Radium Ther. 1948;59:260–81.
Menconi F, Marcocci C, Marinò M. Diagnosis and classification of Graves’ disease. Autoimmun Rev. 2014;13:398–402.
Burch HB, Cooper DS. Management of Graves disease: a review. JAMA. 2015;314:2544–54.
Bartalena L, Burch HB, Burman KD, et al. A 2013 European survey of clinical practice patterns in the management of Graves’ disease. Clin Endocrinol. 2016;84:115–20.
Shi GM, Xu Q, Zhu CY, et al. Influence of propylthiouracil and methimazole pre-treatment on the outcome of iodine-131 therapy in hyperthyroid patients with Graves’ disease. J Int Med Res. 2009;37:576–82.
Urbannek V, Voth E, Moka D, et al. Radioiodine therapy of Graves’ disease–a dosimetric comparison of various therapy regimens of antithyroid agents. Nuklearmedizin Nucl Med. 2001;40:111–5.
Bonnema SJ, Bennedbaek FN, Veje A, et al. Continuous methimazole therapy and its effect on the cure rate of hyperthyroidism using radioactive iodine: an evaluation by a randomized trial. J Clin Endocrinol Metab. 2006;91:2946–51.
Bonnema SJ, Bartalena L, Toft AD, et al. Controversies in radioiodine therapy: relation to ophthalmopathy, the possible radioprotective effect of antithyroid drugs, and use in large goitres. Eur J Endocrinol. 2002;147:1–11.
Sekulic V, Rajic M, Vlajkovic M, et al. The effect of short-term treatment with lithium carbonate on the outcome of radioiodine therapy in patients with long-lasting Graves’ hyperthyroidism. Ann Nucl Med. 2017;31:744–51.
Płazinska MT, Krolicki L, Bąk M. Lithium carbonate pre-treatment in 131-I therapy of hyperthyroidism. Nucl Med Rev Cent East Eur. 2011;14:3–8.
Dunkelmann S, Kunstner H, Nabavi E, et al. Lithium as an adjunct to radioiodine therapy in Graves’ disease for prolonging the intrathyroidal effective half-life of radioiodine. Useful or not? Nuklearmedizin Nucl Med. 2006;45:213–8 (quiz N51-2).
Oszukowska L, Knapska-Kucharska M, Makarewicz J, et al. The influence of thiamazole, lithium carbonate, or prednisone administration on the efficacy of radioiodine treatment ((131)I) in hyperthyroid patients. Endokrynol Polska. 2010;61:56–61.
Saller B, Fink H, Mann K. Kinetics of acute and chronic iodine excess. Exp Clin Endocrinol Diabetes. 1998;106(Suppl 3):S34–8.
Calissendorff J, Falhammar H. Lugol’s solution and other iodide preparations: perspectives and research directions in Graves’ disease. Endocrine. 2017;58:467–73.
Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol. 2014;10:136–42.
Wolff J, Chaikoff IL. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem. 1948;174:555–64.
Wartofsky L, Ransil BJ, Ingbar SH. Inhibition by iodine of the release of thyroxine from the thyroid glands of patients with thyrotoxicosis. J Clin Invest. 1970;49:78–86.
Eng PH, Cardona GR, Fang SL, et al. Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology. 1999;140:3404–10.
Vagenakis AG, Braverman LE. Adverse effects of iodides on thyroid function. Med Clin N Am. 1975;59:1075–88.
Ali A, Debono M, Balasubramanian SP. Outcomes after urgent thyroidectomy following rapid control of thyrotoxicosis in Graves’ disease are similar to those after elective surgery in well-controlled disease. World J Surg. 2019;43:3051–8.
Hassan I, Danila R, Aljabri H, et al. Is rapid preparation for thyroidectomy in severe Graves’ disease beneficial? The relationship between clinical and immunohistochemical aspects. Endocrine. 2008;33:189–95.
Tsai CH, Yang PS, Lee JJ, et al. Effects of preoperative iodine administration on thyroidectomy for hyperthyroidism: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2019;160:993–1002.
Calissendorff J, Falhammar H. Rescue pre-operative treatment with Lugol’s solution in uncontrolled Graves’ disease. Endocr Connect. 2017;6:200–5.
Fischli S, Lucchini B, Muller W, et al. Rapid preoperative blockage of thyroid hormone production / secretion in patients with Graves’ disease. Swiss Med Wkly. 2016;146: w14243.
Huang SM, Liao WT, Lin CF, et al. Effectiveness and mechanism of preoperative Lugol solution for reducing thyroid blood flow in patients with euthyroid graves’ disease. World J Surg. 2016;40:505–9.
Kyrilli A, Tang BN, Huyge V, et al. Thiamazole pretreatment lowers the (131)I activity needed to cure hyperthyroidism in patients with nodular goiter. J Clin Endocrinol Metab. 2015;100:2261–7.
Subramanian M, Baby MK, Seshadri KG. The effect of prior antithyroid drug use on delaying remission in high uptake Graves’ disease following radioiodine ablation. Endocr Connect. 2016;5:34–40.
Zhang R, Zhang G, Wang R, et al. Prediction of thyroidal (131)I effective half-life in patients with Graves’ disease. Oncotarget. 2017;8:80934–40.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MZ and WR conceived and designed the study. CJ performed the experiments and wrote the paper. ZR, ZW, ZG, TJ and JQ reviewed and edited the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The ethical, methodological and protocol aspects of this study were reviewed and approved by the institutional review board and ethic committee of Tianjin Medical University General Hospital. Written informed consents were provided by the participants. Every patient participated in our study voluntarily and comprehended all aspects about the research.
Consent for publication
All authors consent to the publication of this work.
Competing interests
The authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chai, J., Zhang, R., Zheng, W. et al. Effect of Lugol’s solution on 131I therapy efficacy in Graves’ disease. Clin Exp Med 23, 825–831 (2023). https://doi.org/10.1007/s10238-022-00859-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00859-4