Abstract
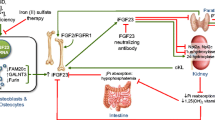
In comparison to the regulation of calcium homeostasis, which has been widely studied over the last several decades, phosphate homeostasis is little understood. The parathyroid hormone (PTH)/vitamin D axis has traditionally been used as a conceptual framework for understanding mineral metabolism. Recently, the fundamental regulator of phosphate homeostasis, fibroblast growth factor 23 (FGF23), which is produced by osteocytes and is involved in the hormonal bone-parathyroid-kidney axis, has attracted more attention. The secretion of FGF23 is controlled by diet, serum phosphate levels, PTH, and 1,25(OH)2 vitamin D. FGF-23, the FGF receptors and the obligate co-receptor α-Klotho work in concert to affect FGF-23 actions on targeted organs. Despite all efforts to investigate pleotropic effects of FGF23 in various endocrine organs, many aspects of the regulation and functions of FGF23 and the exact crosstalk among FGF23, serum phosphate, calcium, PTH, and vitamin D in the regulation of mineral homeostasis remain unclear; much efforts need to be established before it can be moved toward therapeutic applications. In this regard, we provide a brief overview of the novel findings in the regulation and function of FGF23 and refer to related questions and hypotheses not answered yet, which can be a window for future projects. We also focus on the current knowledge about the role of FGF23 obtained from our researches in recent years.




Similar content being viewed by others
Abbreviations
- FGF23:
-
Fibroblast growth factor 23
- FGFR:
-
Fibroblast growth factor receptor
- PTH:
-
Parathyroid hormone
- 1,25-vit D:
-
1,25-Dihydroxy vitamin D3
- ADHR:
-
Autosomal dominant hypophosphatemic rickets
- TIO:
-
Tumor induced-osteomalacia
- CKD:
-
Chronic kidney disease
- ADAM17:
-
Metaloproteinase
- ROMK1:
-
Renal outer medullary potassium channel
- Npt2a/c:
-
Na+‐phosphate cotransporters
- TRPV5:
-
Transient receptor potential cation channel subfamily V member 5
- TRPC6:
-
Transient receptor potential cation channel subfamily C member
- NF-ҡβ:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- p38MAPK:
-
Serine/threonine kinase p38 mitogen-activated protein kinase
- PKC:
-
Serine/threonine kinase protein kinase C
- LPS:
-
Lipopolysaccharide
- IL-1β:
-
Interleukin 1β
- TNFα:
-
Tumor necrosis factor alpha
- TWEAK:
-
The tumor necrosis factor like weak inducer of apoptosis
- PAI-1:
-
Plasminogen activator inhibitor-1
- PLCγ1:
-
Phospholipase Cγ1
- NFAT:
-
Nuclear factor that activates T cells
- GD:
-
Graves’ disease
References
Renkema KY, Alexander RT, Bindels RJ, et al. Calcium and phosphate homeostasis: concerted interplay of new regulators. Ann Med. 2008;40(2):82–91.
Musgrove J, Wolf M. Regulation and effects of FGF23 in chronic kidney disease. Annu Rev Physiol. 2020;82:365–90.
Meyer RA Jr, Meyer MH, Gray RW, et al. Parabiosis suggests a humoral factor is involved in X-linked hypophosphatemia in mice. J Bone Miner Res. 1989;4(4):493–500.
Collins M. Burosumab: at long last, an effective treatment for FGF23-associated hypophosphatemia. J Bone Miner Res. 2018;33(8):1381–2.
Amanzadeh J, Reilly RF. Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nat Clin Pract Nephrol. 2006;2(3):136–48.
Latko M, Czyrek A, Porębska N, et al. Cross-talk between fibroblast growth factor receptors and other cell surface proteins. Cells. 2019;8(5):455.
Dolegowska K, Marchelek-Mysliwiec M, Nowosiad-Magda M, et al. FGF19 subfamily members: FGF19 and FGF21. J Physiol Biochem. 2019;75(2):229–40.
Zou D, Wu W, He Y, et al. The role of klotho in chronic kidney disease. BMC Nephrology. 2018;19(1):1–12.
Krick S, Grabner A, Baumlin N, et al. Fibroblast growth factor 23 and Klotho contribute to airway inflammation. Eur Respir J. 2018;52(1):1800236.
Erben RG. Physiological actions of fibroblast growth factor-23. Front Endocrinol. 2018;9:267.
Shardell M, Semba RD, Kalyani RR, et al. Plasma klotho and frailty in older adults: findings from the InCHIANTI study. J Gerontol Ser A. 2019;74(7):1052–7.
Ohnishi M, Nakatani T, Lanske B, et al. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1α-hydroxylase. Kidney Int. 2009;75(11):1166–72.
Dalton G, An SW, Al-Juboori SI, et al. Soluble klotho binds monosialoganglioside to regulate membrane microdomains and growth factor signaling. Proc Natl Acad Sci. 2017;114(4):752–7.
Scholze A, Liu Y, Pedersen L, et al. Soluble α-klotho and its relation to kidney function and fibroblast growth factor-23. J Clin Endocrinol Metab. 2014;99(5):E855-61.
Akimoto T, Yoshizawa H, Watanabe Y, et al. Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrology. 2012;13(1):1–9.
Smith RC, O’Bryan LM, Farrow EG, et al. Circulating αKlotho influences phosphate handling by controlling FGF23 production. J Clin Investig. 2012;122(12):4710–5.
Ramez M, Rajabi H, Ramezani F, et al. The greater effect of high-intensity interval training versus moderate-intensity continuous training on cardioprotection against ischemia-reperfusion injury through Klotho levels and attenuate of myocardial TRPC6 expression. BMC Cardiovasc Disord. 2019;19(1):1–10.
Chang Q, Hoefs S, van der Kemp AW, et al. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310(5747):490–3.
Dalton GD, Xie J, An SW, et al. New insights into the mechanism of action of soluble klotho. Front Endocrinol. 2017;8:323.
Rhee Y, Bivi N, Farrow E, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49(4):636–43.
Minamizaki T, Konishi Y, Sakurai K, et al. Soluble Klotho causes hypomineralization in Klotho-deficient mice. J Endocrinol. 2018;237(3):285–300.
Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22(1):124–36.
Hu MC, Kuro-o M, Moe OW. Renal and extrarenal actions of Klotho. In: Seminars in nephrology, vol 33, issue 2. Elsevier; 2013. p. 118–129.
Mace ML, Gravesen E, Hofman-Bang J, et al. Key role of the kidney in the regulation of fibroblast growth factor 23. Kidney Int. 2015;88(6):1304–13.
Tang R, Lu Y, Yin R, et al. The effects of storage time and repeated freeze-thaw cycles on intact fibroblast growth factor 23 levels. Biopreserv Biobank. 2021;19(1):48–52.
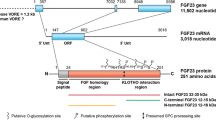
Tagliabracci VS, Engel JL, Wiley SE, et al. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci USA. 2014;111(15):5520–5.
de Las Rivas M, Daniel EJP, Narimatsu Y, et al. Molecular basis for fibroblast growth factor 23 O-glycosylation by GalNAc-T3. Nat Chem Biol. 2020;16(3):351–60.
van Ballegooijen AJ, Rhee EP, Elmariah S, et al. Renal clearance of mineral metabolism biomarkers. J Am Soc Nephrol. 2016;27(2):392–7.
Fukumoto S, Martin TJ. Bone as an endocrine organ. Trends Endocrinol Metab. 2009;20(5):230–6.
Richter M, Polyakova V, Gajawada P, et al. Oncostatin M induces FGF23 expression in cardiomyocytes. J Clin Exp Cardiol. 2012. https://doi.org/10.4172/2155-9880.S9-003.
Egli-Spichtig D, Zhang MY, Perwad F. Fibroblast growth factor 23 expression is increased in multiple organs in mice with folic acid-induced acute kidney injury. Front Physiol. 2018;9:1494.
Kawai M, Kinoshita S, Shimba S, et al. Sympathetic activation induces skeletal Fgf23 expression in a circadian rhythm-dependent manner. J Biol Chem. 2014;289(3):1457–66.
Smith ER, Holt SG, Hewitson TD, et al. αKlotho–FGF23 interactions and their role in kidney disease: a molecular insight. Cell Mol Life Sci. 2019;76(23):4705–24.
Bär L, Stournaras C, Lang F, et al. Regulation of fibroblast growth factor 23 (FGF 23) in health and disease. FEBS Lett. 2019;593(15):1879–900.
Smith ER, McMahon LP, Holt SG. Fibroblast growth factor 23. Ann Clin Biochem. 2014;51(2):203–27.
Damasiewicz MJ, Lu ZX, Kerr PG, et al. The stability and variability of serum and plasma fibroblast growth factor-23 levels in a haemodialysis cohort. BMC Nephrol. 2018;19(1):1–7.
Dirks NF, Smith ER, van Schoor NM, et al. Pre-analytical stability of FGF23 with the contemporary immunoassays. Clin Chim Acta. 2019;493:104–6.
El-Maouche D, Dumitrescu C, Andreopoulou P, et al. Stability and degradation of fibroblast growth factor 23 (FGF23): the effect of time and temperature and assay type. Osteoporos Int. 2016;27(7):2345–53.
Kuro-o M. Klotho and endocrine fibroblast growth factors: markers of chronic kidney disease progression and cardiovascular complications? Nephrol Dial Transplant. 2019;34(1):15–21.
Andrukhova O, Slavic S, Smorodchenko A, et al. FGF 23 regulates renal sodium handling and blood pressure. EMBO Mol Med. 2014;6(6):744–59.
Han X, Cai C, Xiao Z, et al. FGF23 induced left ventricular hypertrophy mediated by FGFR4 signaling in the myocardium is attenuated by soluble Klotho in mice. J Mol Cell Cardiol. 2020;138:66–74.
Han X, Ross J, Kolumam G, et al. Cardiovascular effects of renal distal tubule deletion of the FGF receptor 1 gene. J Am Soc Nephrol. 2018;29(1):69–80.
Perwad F, Zhang MY, Tenenhouse HS, et al. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1α-hydroxylase expression in vitro. Am J Physiol Ren Physiol. 2007;293(5):F1577–83.
Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113(4):561–8.
Andrukhova O, Zeitz U, Goetz R, et al. FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2–SGK1 signaling pathway. Bone. 2012;51(3):621–8.
Takeshita A, Kawakami K, Furushima K, et al. Central role of the proximal tubular αKlotho/FGF receptor complex in FGF23-regulated phosphate and vitamin D metabolism. Sci Rep. 2018;8(1):1–15.
Saki F, Omrani GR, Koohpeyma F. Investigating the effect of paricalcitol on serum FGF23 in vitamin D deficient rats. 2019.
Kamelian T, Saki F, Jeddi M, et al. Effect of Cholecalciferol therapy on serum FGF(23) in vitamin D deficient patients: a randomized clinical trial. J Endocrinol Invest. 2018;41(3):299–306.
Silver J, Naveh-Many T. FGF23 and the parathyroid glands. Pediatric nephrology. 2010;25(11):2241–5.
Kawakami K, Takeshita A, Furushima K, et al. Persistent fibroblast growth factor 23 signalling in the parathyroid glands for secondary hyperparathyroidism in mice with chronic kidney disease. Sci Rep. 2017;7(1):1–14.
Galitzer H, Ben-Dov I, Silver J, et al. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77(3):211–8.
Saki F, Kassaee SR, Salehifar A, et al. Interaction between serum FGF-23 and PTH in renal phosphate excretion, a case-control study in hypoparathyroid patients. BMC Nephrol. 2020;21(1):176.
Yamashita H, Yamashita T, Miyamoto M, et al. Fibroblast growth factor (FGF)-23 in patients with primary hyperparathyroidism. Eur J Endocrinol. 2004;151(1):55–60.
Gutiérrez OM, Smith KT, Barchi-Chung A, et al. (1–34) Parathyroid hormone infusion acutely lowers fibroblast growth factor 23 concentrations in adult volunteers. Clin J Am Soc Nephrol. 2012;7(1):139–45.
Tebben PJ, Singh RJ, Clarke BL, et al., editors. Fibroblast growth factor 23, parathyroid hormone, and 1α, 25-dihydroxyvitamin D in surgically treated primary hyperparathyroidism. In: Mayo Clinic Proceedings. Elsevier; 2004.
Murali SK, Roschger P, Zeitz U, et al. FGF23 regulates bone mineralization in a 1, 25 (OH) 2D3 and klotho-independent manner. J Bone Miner Res. 2016;31(1):129–42.
Carrillo-López N, Panizo S, Alonso-Montes C, et al. Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int. 2016;90(1):77–89.
Iguchi A, Yamamoto S, Yamazaki M, et al. Effect of ferric citrate hydrate on FGF23 and PTH levels in patients with non-dialysis-dependent chronic kidney disease with normophosphatemia and iron deficiency. Clin Exp Nephrol. 2018;22(4):789–96.
Abu-Zaid A, Magzoub D, Aldehami MA, et al. The effect of iron supplementation on FGF23 in chronic kidney disease patients: a systematic review and time-response meta-analysis. Biol Trace Elem Res. 2021;199:4516.
Coe LM, Madathil SV, Casu C, et al. FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. J Biol Chem. 2014;289(14):9795–810.
Madathil SV, Coe LM, Casu C, et al. Klotho deficiency disrupts hematopoietic stem cell development and erythropoiesis. Am J Pathol. 2014;184(3):827–41.
Bär L, Feger M, Fajol A, et al. Insulin suppresses the production of fibroblast growth factor 23 (FGF23). Proc Natl Acad Sci USA. 2018;115(22):5804–9.
Thrailkill KM, Nyman JS, Bunn RC, et al. The impact of SGLT2 inhibitors, compared with insulin, on diabetic bone disease in a mouse model of type 1 diabetes. Bone. 2017;94:141–51.
Gateva A, Tsakova A, Hristova J, et al. Fibroblast growth factor 23 and 25 (OH) D levels are related to abdominal obesity and cardiovascular risk in patients with polycystic ovarian syndrome. Gynecol Endocrinol. 2020;36(5):402–5.
Kan S, Kızılgül M, Culha C, et al. Fibroblast growth factor-23 concentrations in polycystic ovary syndrome. Turk J Biochem. 2018;43(1):83–8.
Lopez I, Pineda C, Raya AI, et al. Leptin directly stimulates parathyroid hormone secretion. Endocrine. 2017;56(3):675–8.
Rutkowski JM, Pastor J, Sun K, et al. Adiponectin alters renal calcium and phosphate excretion through regulation of klotho expression. Kidney Int. 2017;91(2):324–37.
Lubkowska A, Dobek A, Mieszkowski J, et al. Adiponectin as a biomarker of osteoporosis in postmenopausal women: controversies. Dis Mark. 2014;2014:1–14.
Marchelek-Mysliwiec M, Wisniewska M, Nowosiad-Magda M, et al. Association between plasma concentration of klotho protein, osteocalcin, leptin, adiponectin, and bone mineral density in patients with chronic kidney disease. Horm Metab Res. 2018;50(11):816–21.
Tippen SP, Noonan ML, Ni P, et al. Age and sex effects on FGF23-mediated response to mild phosphate challenge. Bone. 2021;146:115885.
Nakamura T, Imai Y, Matsumoto T, et al. Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell. 2007;130(5):811–23.
Forough S, Faezeh S, Kasaee SR, et al. Effect of prolactin and estrogen on the serum level of 1, 25-dihydroxy vitamin D and FGF23 in female rats. Arch Gynecol Obstet. 2020;302(1):265–71.
Wetmore JB. The link between estrogen and fibroblast growth factor 23. Am J Kidney Dis. 2011;58(5):695–6.
Tanaka Y, Castillo L, Wineland MJ, et al. Synergistic effect of progesterone, testosterone, and estradiol in the stimulation of chick renal 25-hydroxyvitamin D3-lα-hydroxylase. Endocrinology. 1978;103(6):2035–9.
Saki F, Dabbaghmanesh MH, Omrani GR, et al. Vitamin D deficiency and its associated risk factors in children and adolescents in southern Iran. Public Health Nutr. 2017;20(10):1851–6.
Dote-Montero M, Amaro-Gahete FJ, Jurado-Fasoli L, et al. Study of the association of DHEAS, testosterone and cortisol with S-Klotho plasma levels in healthy sedentary middle-aged adults. Exp Gerontol. 2019;121:55–61.
Finkelstein JS, Hayes A, Hunzelman JL, et al. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349(13):1216–26.
Finkelstein JS, Wyland JJ, Lee H, et al. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1838–45.
Bøllehuus Hansen L, Kaludjerovic J, Nielsen JE, et al. Influence of FGF23 and Klotho on male reproduction: systemic vs direct effects. FASEB J. 2020;34(9):12436–49.
Saki F, Kasaee S, Sadeghian F, et al. Investigating the effect of testosterone by itself and in combination with letrozole on 1, 25-dihydroxy vitamin D and FGF23 in male rats. J Endocrinol Invest. 2019;42(1):19–25.
Kubota M, Ohno J, Shiina Y, et al. Vitamin D metabolism in pregnant rabbits: differences between the maternal and fetal response to administration of large amounts of vitamin D3. Endocrinology. 1982;110(6):1950–6.
Saki F, Sadeghian F, Kasaee SR, et al. The effect of prolactin itself and in combination with estrogen on bone mineral density in female rats. Gynecol Endocrinol. 2019;35(6):539–43.
Leifheit-Nestler M, Kirchhoff F, Nespor J, et al. Fibroblast growth factor 23 is induced by an activated renin–angiotensin–aldosterone system in cardiac myocytes and promotes the pro-fibrotic crosstalk between cardiac myocytes and fibroblasts. Nephrol Dial Transplant. 2018;33(10):1722–34.
Radloff J, Pagitz M, Andrukhova O, et al. Aldosterone is positively associated with circulating FGF23 levels in chronic kidney disease across four species, and may drive FGF23 secretion directly. Front Physiol. 2021. https://doi.org/10.3389/fphys.2021.649921.
Akhabue E, Vu THT, Vaidya A, et al. Fibroblast growth factor-23, heart failure risk, and renin–angiotensin–aldosterone-system blockade in hypertension: the MESA study. Am J Hypertens. 2019;32(1):18–25.
Hayashi K, Suzuki T, Sakamaki Y, et al. Cardiac hypertrophy in chronic kidney disease—role of Aldosterone and FGF23. Ren Replace Ther. 2018;4(1):1–12.
Wang W, Yinbiao S, Jian X, et al. Elevated serum concentrations of FGF23 and Klotho in patients with Graves disease and their significance. Clin Med China. 2018;34(6):481–4.
Lin C-H, Chang C-K, Shih C-W, et al. Serum fibroblast growth factor 23 and mineral metabolism in patients with euthyroid Graves’ diseases: a case-control study. Osteoporos Int. 2019;30(11):2289–97.
Acknowledgements
We dedicate this article to Prof. Gholam Hossein Ranjbar Omrani, who passed away recently and had the leading role in all steps of this project. He was a pioneer and a world-class scientist in endocrine and metabolism researches. We tried to gather all his novel findings in the topic of FGF23 for a bit of appreciation for their tireless efforts in training junior researchers for many years. Hereby, the authors would like to thank Dr. Manica Negahdaripour for English editing of the manuscript.
Funding
There is no financial support.
Author information
Authors and Affiliations
Contributions
SD: did complete searching to gather all related papers, and prepared the first draft of the manuscript, FK: prepared the second draft of the manuscript, AA: made the initial plan of the project, read the second draft, and revised article, FS: made the final plan for the project, did a final proof of manuscript, and the was the corresponding author; MS: made the initial plan of the project, and revised the final draft of manuscript and the correspondence. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dastghaib, S., Koohpeyma, F., Shams, M. et al. New concepts in regulation and function of the FGF23. Clin Exp Med 23, 1055–1066 (2023). https://doi.org/10.1007/s10238-022-00844-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00844-x