Abstract
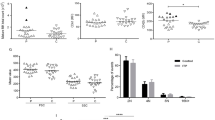
Both platelet count and function change after treatment of immune thrombocytopenia. Platelet function can be measured by plasma markers, including platelet activity [e.g., soluble P-selectin (sP-selectin) and soluble CD40 ligand (sCD40L)] and platelet turnover markers [e.g., glycocalicin (GC)]. Patients were classified into no response (NR, including new diagnosis), partial response (PR) and complete response (CR). One hundred and sixteen samples (29 CR, 32 PR, 55 NR) from 79 patients were collected. Plasma markers (sP-selectin, sCD40L and GC) were measured by ELISA. Platelet counts and mean platelet volume (MPV) were obtained in the clinical laboratory using GenS System-2. The results showed that responsive patients (PR + CR) had higher levels of sP-selectin (P = 0.026) and sCD40L (P = 0.001). Although there was no difference in MPV (P = 0.077) or GC (P = 0.078), there was a marked decrease of GC index (P < 0.001) in responsive patients. Paired sample analysis showed no difference in sP-selectin, sCD40L, MPV or GC but significant difference in GC index (P = 0.017) between NR and PR. Another paired sample analysis showed no difference in sP-selectin, sCD40L, MPV or GC but significant difference in GC index (P = 0.029) between PR and CR. Patients with refractory and newly diagnosed disease had a significant difference in GC (P = 0.020) and sCD40L (P = 0.001), despite similarly low platelet counts. In conclusion, platelet activity markers (sP-selectin and sCD40L) and GC indices change in parallel with treatment response. Plasma levels of GC and sCD40L may be predictors of treatment response.







Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Skipper MT, Rubak P, Stentoft J, Hvas AM, Larsen OH. Evaluation of platelet function in thrombocytopenia. Platelets. 2017. https://doi.org/10.1080/09537104.2017.1296566.
Skipper MT, Rubak P, Stentoft J, Hvas AM, Larsen OH. Evaluation of platelet function in thrombocytopenia. Platelets. 2018;29(3):270–6. https://doi.org/10.1080/09537104.2017.1296566.
Panzer S, Hocker L, Vormittag R, Rieger M, Koren D, Dunkler D, Pabinger I. Flow cytometric evaluation of platelet activation in chronic autoimmune thrombocytopenia. Pediatr Blood Cancer. 2006;47(5 Suppl):694–6. https://doi.org/10.1002/pbc.21000.
Chong BH, Murray B, Berndt MC, Dunlop LC, Brighton T, Chesterman CN. Plasma P-selectin is increased in thrombotic consumptive platelet disorders. Blood. 1994;83(6):1535–41.
Haznedaroglu IC, Buyukasik Y, Kosar A, Kirazh S, Dundar SV. Thrombopoietin, interleukin-6, and P-selectin at diagnosis and during post-steroid recovery period of patients with autoimmune thrombocytopenic purpura. Ann Hematol. 1998;77(4):165–70.
Olcay L, Yenicesu I, Yetgin S. Soluble P-selectin, interleukin 6, and thrombopoietin levels in children with acute and chronic idiopathic thrombocytopenic purpura and their relationship with mega-dose methylprednisolone therapy: a pilot study. J Pediatr Hematol Oncol. 2002;24(9):742–5.
Louwes H, Zeinali Lathori OA, Vellenga E, de Wolf JT. Platelet kinetic studies in patients with idiopathic thrombocytopenic purpura. Am J Med. 1999;106(4):430–4. https://doi.org/10.1016/s0002-9343(99)00054-6.
Rossi G, Cattaneo C, Motta M, Pizzocaro C, Lanzi S, Pouche A. Platelet kinetic study in patients with idiopathic thrombocytopenic purpura (ITP) refractory or relapsing after corticosteroid treatment. Hematol J Off J Eur Haematol Assoc. 2002;3(3):148–52. https://doi.org/10.1038/sj.thj.6200170.
Beer JH, Buchi L, Steiner B. Glycocalicin: a new assay—the normal plasma levels and its potential usefulness in selected diseases. Blood. 1994;83(3):691–702.
Bessos H, Murphy WG, Seghatchian MJ. Monitoring the release of glycocalicin in platelet concentrates by ELISA. Blood Coagul Fibrinolysis Int J Haemost Thromb. 1991;2(2):373–6.
Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N, Godeau B, Lechner K, Mazzucconi MG, McMillan R, Sanz MA, Imbach P, Blanchette V, Kuhne T, Ruggeri M, George JN. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–93. https://doi.org/10.1182/blood-2008-07-162503.
Al-Samkari H, Van Cott EM, Kuter DJ. Platelet aggregation response in immune thrombocytopenia patients treated with romiplostim. Ann Hematol. 2019;98(3):581–8. https://doi.org/10.1007/s00277-018-3556-6.
Frelinger AL 3rd, Grace RF, Gerrits AJ, Berny-Lang MA, Brown T, Carmichael SL, Neufeld EJ, Michelson AD. Platelet function tests, independent of platelet count, are associated with bleeding severity in ITP. Blood. 2015. https://doi.org/10.1182/blood-2015-02-628461.
Middelburg RA, Carbaat-Ham JC, Hesam H, Ragusi MA, Zwaginga JJ. Platelet function in adult ITP patients can be either increased or decreased, compared to healthy controls, and is associated with bleeding risk. Hematology. 2016;21(9):549–51. https://doi.org/10.1080/10245332.2016.1180097.
Panzer S, Rieger M, Vormittag R, Eichelberger B, Dunkler D, Pabinger I. Platelet function to estimate the bleeding risk in autoimmune thrombocytopenia. Eur J Clin Invest. 2007;37(10):814–9. https://doi.org/10.1111/j.1365-2362.2007.01855.x.
Kurata Y, Hayashi S, Kiyoi T, Kosugi S, Kashiwagi H, Honda S, Tomiyama Y. Diagnostic value of tests for reticulated platelets, plasma glycocalicin, and thrombopoietin levels for discriminating between hyperdestructive and hypoplastic thrombocytopenia. Am J Clin Pathol. 2001;115(5):656–64. https://doi.org/10.1309/raw2-0lqw-8ytx-941v.
Thomas-Kaskel AK, Mattern D, Kohler G, Finke J, Behringer D. Reticulated platelet counts correlate with treatment response in patients with idiopathic thrombocytopenic purpura and help identify the complex causes of thrombocytopenia in patients after allogeneic hematopoietic stem cell transplantation. Cytometry B Clin Cytom. 2007;72(4):241–8. https://doi.org/10.1002/cyto.b.20163.
Houwerzijl EJ, Louwes H, Esselink MT, Smit JW, Vellenga E, de Wolf JT. Increased glycocalicin index and normal thrombopoietin levels in patients with idiopathic thrombocytopenic purpura with a decreased rate of platelet production. Haematologica. 2005;90(5):710–1.
Khaspekova SG, Shustova ON, Golubeva NV, Vasiliev SA, Mazurov AV. Relationships of mean platelet volume and plasma thrombopoietin with glycocalicin levels in thrombocytopenic patients. Acta Haematol. 2015;133(3):295–9. https://doi.org/10.1159/000362531.
Kunishima S, Tahara T, Kato T, Kobayashi S, Saito H, Naoe T. Serum thrombopoietin and plasma glycocalicin concentrations as useful diagnostic markers in thrombocytopenic disorders. Eur J Haematol. 1996;57(1):68–71.
Lyu ME, Li Y, Lyu CC, Liu WJ, Guan Y, Wang SX, Yang RC (2017) [Relative analysis of platelet activation with bleeding risk in patients with primary immune thrombocytopenia]. Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi 38 (1):33–38. https://doi.org/10.3760/cma.j.issn.0253-2727.2017.01.007
Psaila B, Bussel JB, Frelinger AL, Babula B, Linden MD, Li Y, Barnard MR, Tate C, Feldman EJ, Michelson AD. Differences in platelet function in patients with acute myeloid leukemia and myelodysplasia compared to equally thrombocytopenic patients with immune thrombocytopenia. J Thromb Haemos JTH. 2011;9(11):2302–10. https://doi.org/10.1111/j.1538-7836.2011.04506.x.
Barsam SJ, Psaila B, Forestier M, Page LK, Sloane PA, Geyer JT, Villarica GO, Ruisi MM, Gernsheimer TB, Beer JH, Bussel JB. Platelet production and platelet destruction: assessing mechanisms of treatment effect in immune thrombocytopenia. Blood. 2011;117(21):5723–32. https://doi.org/10.1182/blood-2010-11-321398.
Frelinger AL 3rd, Grace RF, Gerrits AJ, Carmichael SL, Forde EE, Michelson AD. Platelet Function in ITP, independent of platelet count, is consistent over time and is associated with both current and subsequent bleeding severity. Thromb Haemost. 2018;118(1):143–51. https://doi.org/10.1160/th17-06-0387.
Nagahama M, Nomura S, Kanazawa S, Ozaki Y, Kagawa H, Fukuhara S. Significance of chemokines and soluble CD40 ligand in patients with autoimmune thrombocytopenic purpura. Eur J Haematol. 2002;69(5–6):303–8.
Meabed MH, Taha GM, Mohamed SO, El-Hadidy KS. Autoimmune thrombocytopenia: flow cytometric determination of platelet-associated CD154/CD40L and CD40 on peripheral blood T and B lymphocytes. Hematology. 2007;12(4):301–7. https://doi.org/10.1080/10245330701383957.
Patel VL, Schwartz J, Bussel JB. The effect of anti-CD40 ligand in immune thrombocytopenic purpura. Br J Haematol. 2008;141(4):545–8. https://doi.org/10.1111/j.1365-2141.2008.07039.x.
Lyu M, Li Y, Xue F, Liu X, Liu W, Sun T, Lyu C, Fu R, Zhang L, Yang R (2015) [Application of immature platelet fractionabsolute immature platelet fraction and thrombelastograph on assessment of bleeding risk in patients with immune thrombocytopenia]. Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi 36 (9):759–764. https://doi.org/10.3760/cma.j.issn.0253-2727.2015.09.008
Meyer O, Herzig E, Salama A. Platelet kinetics in idiopathic thrombocytopenic purpura patients treated with thrombopoietin receptor agonists. Transf Med Hemother Offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2012;39(1):5–8. https://doi.org/10.1159/000335553.
Funding
This study was supported by grant NMRPG3F0521 awarded by Ministry of Science and Technology, Taiwan.
Author information
Authors and Affiliations
Contributions
CO and HC designed the study and wrote this manuscript. Y-SH, M-CK and T-LL collected patient information and revised the manuscript. P-LL conducted the experiment and analyzed the data. All authors reviewed and approved the contents of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declared they have no conflict of interest.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board of Chang Gung Memorial Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ou, C., Chang, H., Hung, YS. et al. Changing profile of platelet activity and turnover indices during treatment response of immune thrombocytopenia. Clin Exp Med 22, 595–603 (2022). https://doi.org/10.1007/s10238-022-00790-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-022-00790-8