Abstract
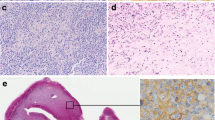
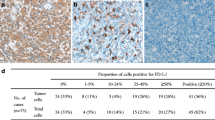
Epstein–Barr virus (EBV)+ diffuse large B-cell lymphoma (DLBCL) has specific tumour cell characteristics, and these patients have worse outcomes than EBV-negative DLBCL patients. We compared 38 EBV+ DLBCL patients with 43 methotrexate-associated EBV+ B-cell lymphoproliferative disorders (MTX+/EBV+ BLPDs) and 30 non-germinal centre (GC) subtype DLBCL. Lymphoma cells of the EBV+ DLBCL group were positive for BCL2 in 17 patients (44.7%), CMYC in 23 patients (60.5%), and p53 in 33 patients (86.8%), which was significantly higher than in the MTX+/EBV+ BLPD group (P < 0.05), and were positive for CD30 in 29 patients (76.3%), compared with two in non-GC subtype DLBCL (6.7%) (P < 0.0001). Significantly more EBV+ DLBCL patients (n = 16, 42.1%) had programmed cell death-ligand 1 (PD-L1)+ tumour cells than patients with non-GC subtype DLBCL (n = 5, 16.7%; P = 0.024), and PD-L1+ tumour cells were more common in advanced stages than in early stages (P = 0.048). Twenty-five EBV+ DLBCL patients (69.4%) had few reactive PD1+ tumour-infiltrating lymphocytes (TILs), compared with 12 patients with MTX+/EBV+ BLPDs (37.5%) (P = 0.008). In the EBV+ DLBCL group, CD30, BCL2, CMYC, and p53 expression was not related to patient prognosis. Poor outcomes were associated with PD-L1+ tumour cells (P = 0.001) and low-reacting PD1+ TILs (P = 0.02), while their combination conferred a worse outcome (P < 0.0001). Immune evasion by PD-L1+ tumour cells and exhaustion of PD1+ TILs may occur in EBV+ DLBCL patients, and PD-L1/PD1 interactions may influence tumour progression and poor prognosis.



Similar content being viewed by others
References
Nakamura S, Jaffe ES, Swerdlow S, et al. EBV-positive diffuse large B-cell lymphoma, not otherwise specified (NOS). In: Swerdlow S, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues, revised. 4th ed. Lyon: IARC Press; 2017. p. 304–6.
Gaulard P, Swerdlow S, Harris N, et al. Other iatrogenic immunodeficiency-associated lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues, revised. 4th ed. Lyon: IARC Press; 2017. p. 462–4.
Oyama T, Yamamoto K, Asano N, et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a studyof 96 patients. Clin Cancer Res. 2007;13:5124–32.
Ok CY, Ye Q, Li L, et al. Age cutoff for Epstein–Barr virus-positive diffuse large B-cell lymphoma—is it necessary? Oncotarget. 2015;6:13933–45.
Montes-moreno S, Odqvist L, Diaz-perez JA, et al. EBV-positive diffuse large B-cell lymphoma of the elderly is an aggressive post-germinal center B-cell neoplasm characterized by prominent nuclear factor-κB activation. Mod Pathol. 2012;25:968–82.
Ok CY, Li L, Xu-monette ZY, et al. Prevalence and clinical implications of Epstein-Barr virus infection in de novo diffuse large B-cell lymphoma in western countries. Clin Cancer Res. 2014;20:2338–49.
Ejima-yamada K, Oshiro Y, Okamura S, et al. Epstein-Barr virus infection and gene promoter hypermethylation in rheumatoid arthritis patients with methotrexate-associated B cell lymphoproliferative disorders. Virchows Arch. 2017;470:205–15.
Wang XJ, Medeiros LJ, Bueso-Ramos CE, et al. P53 expression correlates with poorer survival and augments the negative prognostic effect of MYC rearrangement, expression or concurrent MYC/BCL2 expression in diffuse large B-cell lymphoma. Mod Pathol. 2017;30:194–203.
Xie Y, Ajaz Bulbul M, Ji L, et al. P53 expression is a strong marker of inferior survival in de novo diffuse large B-cell lymphoma and may have enhanced negative effect with MYC coexpression: a single institutional clinicopathologic study. Am J Clin Pathol. 2014;141:593–604.
Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126:2193–201.
Kwon D, Kim S, Kim PJ, et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B-cell lymphomas. Histopathology. 2016;68:1079–89.
Xu-monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131:68–84.
Song MK, Park BB, Uhm J. Understanding immune evasion and therapeutic targeting associated with PD-1/PD-L1 pathway in diffuse large B-cell lymphoma. Int J Mol Sci. 2019;20:1–15.
Fang X, Xiu B, Yang Z, et al. The expression and clinical relevance of PD-1, PD-L1, and TP63 in patients with diffuse large B-cell lymphoma. Medicine. 2017;96:1–7.
Anastasiadou E, Stroopinsky D, Alimperti S, et al. Epstein−Barr virus-encoded EBNA2 alters immune checkpoint PD-L1 expression by downregulating miR-34a in B-cell lymphomas. Leukemia. 2019;33:132–47.
Stuhlmann-Laeisz C, Borchert A, Quintanilla-Martinez L, et al. In Europe expression of EBNA2 is associated with poor survival in EBV-positive diffuse large B-cell lymphoma of the elderly. Leuk Lymphoma. 2016;57:39–44.
Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;2:3462–74.
Sakakibara A, Kohno K, Eladl AE, et al. Immunohistochemical assessment of the diagnostic utility of PD-L1: a preliminary analysis of anti-PD-L1 antibody (SP142) for lymphoproliferative diseases with tumour and non-malignant Hodgkin–Reed–Sternberg (HRS)-like cells. Histopathology. 2018;72:1156–63.
Yoon H, Sanghui P, Hyunjeong J, et al. Integrated copy number and gene expression profiling analysis of Epstein–Barr virus-positive diffuse large B-cell lymphoma. Genes Chromosomes Cancer. 2015;396:389–96.
Takahara T, Satou A, Ishikawa E, et al. Clinicopathological analysis of neoplastic PD-L1-positive EBV+ diffuse large B cell lymphoma, not otherwise specified, in a Japanese cohort. Virchows Arch. 2020. https://doi.org/10.1007/s00428-020-02901-w.
Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018;50:74–87.
Witte HM, Merz H, Biersack H, et al. Impact of treatment variability and clinicopathological characteristics on survival in patients with Epstein-Barr-virus positive diffuse large B cell lymphoma. Brit J Haematol. 2020;189:257–68.
Zhou Y, Xu Z, Lin W, et al. Comprehensive genomic profiling of EBV-positive diffuse large B-cell lymphoma and the expression and clinicopathological correlations of some related genes. Front Oncol. 2019. https://doi.org/10.3389/fonc.2019.00683.
Veloza L, Teixido C, Castrejon N, et al. Clinicopathological evaluation of the programmed cell death 1 (PD1)/programmed cell death-ligand 1 (PD-L1) axis in post-transplant lymphoproliferative disorders: association with Epstein-Barr virus, PD-L1 copy number alterations, and outcome. Histopathology. 2019;75:799–812.
Keane C, Tobin J, Gunawardana J, et al. The tumour microenvironment is immuno-tolerogenic and a principal determinant of patient outcome in EBV-positive diffuse large B-cell lymphoma. Eur J Haematol. 2019;103:200–7.
Gravelle P, Burroni B, Péricart S, et al. Mechanisms of PD-1/PD-L1 expression and prognostic relevance in non-Hodgkin lymphoma: a summary of immunohistochemical studies. Oncotarget. 2017;8:44960–75.
Cohen M, Vistarop AG, Huaman F, et al. Cytotoxic response against Epstein Barr virus coexists with diffuse large B-cell lymphoma tolerogenic microenvironment: clinical features and survival impact. Sci Rep. 2017;7:1–10.
Kim S, Nam SJ, Park C, et al. High tumoural PD-L1 expression and low PD-1+ or CD8+ tumour-infiltrating lymphocytes are predictive of a poor prognosis in primary diffuse large B-cell lymphoma of the central nervous system. Oncoimmunology. 2019;8:1–10.
Pascual M, Mena-Varas M, Robles EF, et al. PD-1/PD-L1 immune checkpoint and p53 loss facilitate tumor progression in activated B-cell diffuse large B-cell lymphoma. Blood. 2019;133:2401–12.
Carreras J, Yara Yukie Kikuti YY, Miyaoka M, et al. Genomic profile and pathologic features of diffuse large B-cell lymphoma subtype of methotrexate-associated lymphoproliferative disorder in rheumatoid arthritis patients. Am J Surg Pathol. 2018;42:936–50.
Ichikawa A, Arakawa F, Kiyasu J, et al. Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: histology, Epstein–Barr virus, and clonality are important predictors of disease progression and regression. Eur J Haematol. 2013;91:20–8.
Acknowledgements
We are grateful to Tomomi Okabe and Tomoko Fukushige for technical assistance with the immunohistochemistry assays. We also thank H. Nikki March, Ph.D, from Edanz (https://jp.edanz.com/ac), for editing a draft of this manuscript.
Funding
This study was supported in part by a Grant-in-Aid for Scientific Research (No. 17K08732) from the Ministry of Education, Science, and Culture of Japan.
Author information
Authors and Affiliations
Contributions
SK, YM, and MT contributed to the experimental design. HI, MK, YM, TS, and YT retrieved and reviewed the clinical data. YO, ZW, SK, and MT contributed to the histological diagnosis and collected patient samples. YM and MT performed the IHC scoring. Statistical analysis was performed by TS. The manuscript was written by SK and MT and approved by all contributing authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no significant relationships with or financial interests in any commercial activities pertaining to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kimura, S., Oshiro, Y., Iwasaki, H. et al. Programmed cell death-ligand 1 (PD-L1)+ tumour cells and low-reacting programmed cell death 1 (PD1)+ tumour-infiltrating lymphocytes predict poor prognosis in Epstein–Barr virus+ diffuse large B-cell lymphoma. Clin Exp Med 22, 411–419 (2022). https://doi.org/10.1007/s10238-021-00754-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-021-00754-4