Abstract
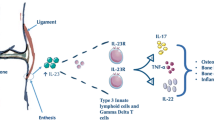
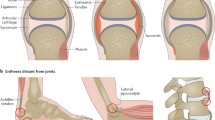
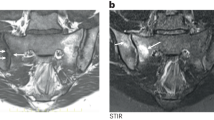
The group of diseases classified as seronegative spondyloarthritis or enthesoarthritis is characterized by typical osteoarticular and extra-articular manifestations. Diverse patterns of disease can affect different members of the same family and may show different features in the same patient, with clinical overlaps thwarting the differential diagnosis. An anatomo-pathological hallmark in enthesoarthritis is the inflammatory process in the synovio-entheseal sites. The inflammatory microenvironment of synovio-entheseal complex, named enthesitis, is characterized, after an initial inflammatory/erosive phase, by a subsequent phase of neobone apposition, which seems to progress independently from the previous erosive phase, suggesting that the physiopathogenetic mechanisms that underlay the two phases are driven by different pivots. The structural damage is characterized by excessive neobone formation, with the syndesmophyte as a typical lesion. The process underlying their formation is not fully understood, although there are many useful information to clarify the physiopathogenetic puzzle. The primum movens of the enthesitic process is the micro-trauma to which entheses are subject, especially in the lower limbs, for biomechanical reasons. The inflammatory process is facilitated by the sequential structure of the organ enthesis, constitutionally devoid of sub-enthesitic cortical bone and closely related to the underlying trabecular bone and the medullary vascular system. The reparative attempt from the vascular system, thanks to the activating action of certain loco-regional cytokines, such as TNF α, conditions the possible deposit in the enthesis of molecules derived from other organic sites and able, especially in HLA-B27+ subjects, to activate and self-renew an immune-mediated inflammatory process following the initial mechanical process.




Similar content being viewed by others
References
Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2011;199:503–26.
Benjamin M. The enthesis organ concept and its relevance to the spondyloarthropathies. In: Lopez-Larrea C, Diaz-Pena R, editors. Molecular mechanisms of spondyloarthropathies, vol. 4. New York: Landes Bioscience and Springer Sciences Business Media; 2009. p. 57–70.
Benjamin M, Toumi H, Suzuki D, Redman S, Emery P, McGonagle D. Microdamage and altered vascularity at the enthesis-bone interface provides an anatomic explanation for Bone involvement in the HLA-B-27-associated spondyloarthritides and allied conditions. Arth Rheum. 2007;56(1):224–33.
McGonagle D, Lories RJ, Tan AL, Benjamin M. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arth Rheum. 2007;56:2482–91.
Kahn MF, Chamot AM. SAPHO syndrome. Rheum Dis Clin North Am. 1992;18:225–46.
Chamot AM, Benhamou CL, Kahn MF, Beraneck L, Kaplan G, Prost A. Acne-pustolosis-hyperostosis-osteitis syndrome: results of a National survey, 85 cases (review). Rev Rhum Mal Osteoartic. 1987;54:187–96.
McGonagle D, Stockwin L, Isaacs J, Emery P. An enthesitis based model for the pathogenesis of spondyloathropathy: additive effects of microbial adjuvant and biomechanical factors at disease sites. J Rheumatol. 2001;28:2155–9.
Maksymowych WP. Ankylosing spondylitis: at the interface of bone and cartilage. J Rheumatol. 2000;27:2295–301.
McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Med. 2006;3(8):e297.
Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005;115:1571–9.
Lories RJ, Daans M, Derese I, et al. Nogging haploinsufficiency differentially affects tissue responses in destructive and remodeling arthritis. Arth Rheum. 2006;54:1736–46.
Lories RJ, Derese I, de Bari C, Luyten FP. Evidence for uncoupling of inflammation and joint remodelling in a mouse model of spondylarthritis. Arth Rheum. 2007;56:489–97.
Lories RJ, Derese I, Luyten FP. Inhibition of osteoclast does not prevent joint ankylosis in a mouse model of spondyloartrhritis. Rheumatology. 2008;47:605–8.
Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodelling. Nat Med. 2007;13:156–63.
Uderhardt S, Diarra D, Katzenbeisser J, et al. Blockade of Dickkopf (DKK)-1 induces fusion of sacroiliac joints. Ann Rheum Dis. 2010;69(3):592–7. doi:10.1136/ard.2008.102046.
Keffer J, Probert L, Cazlaris H, et al. Transgenic mice expressing human tumor necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–31.
Shealy DJ, Wooley PH, Emmell E, et al. Anti-TNF alpha antibody allows healing of joint damage in polyarthritic transgenic mice. Arthritis Res. 2002;4:R7.
Pettit AR, Ji H, von Stechow D, et al. TRANCE/RANKL knockout mice are protected from bone erosionin a serum transfer model of arthritis. Am J Pathol. 2001;159:1689–99.
Haynes DR, Barg E, Crotti TN, et al. Osteoprotegerin expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathies and osteoarthritis and normal controls. Rheumatology. 2003;42:123–34.
Vandooren B, Cantaert T, Noordenbos T, Tak PP, Baeten D. The abundant synovial expression of the RANK/RANKL/Osteoprotegerin system in peripheral spondylarthritis is partially disconnected from inflammation. Arthritis Rheum. 2008;58:718–29.
Kotake S, Udagawa N, Hakoda M, et al. Activated human T cells directly induce osteoclastogenesis from human monocytis: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. 2001;44:1003–12.
Crotti TN, Flannery M, Walsh NC, Fleming JD, Goldring SR, McHugh KP. NFATc1 directly induces the human beta3 integrin gene in osteoclast differentiation. J Muscoloskeletal Neuronal Interact. 2005;5:335–7.
Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–46.
Crotti TN, Sharma SM, Fleming JD, et al. PU.1 and NFATc1 mediate osteoclastic induction of the mouse beta3 integrin promoter. J Cell Physiol. 2008;215:636–44.
Shen Z, Crotti TN, Flannery MR, Matsuzaki K, Goldring SR, McHugh KP. A novel promoter regulates calcitonin receptor gene expression in human osteoclasts. Biochim Biophys Acta. 2007;1769:659–67.
van der Heijde D, Landewé R, Einstein S, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum. 2008;58:1324–31.
van der Heijde D, Landewé R, Baraliakos X, et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum. 2008;58:3063–70.
Van der Heijde D. Adalimumab therapy for ankylosing spondylitis over two years does not demonstrate inhibition of radiographic progression compared with a historical control group. Arthr Rheum 2008;58(Suppl):5413; abstract 670.
Baraliakos X, Listing J, Brandt J, et al. Radiographic progression in patients with ankylosing spondylitis after 4 yrs of treatment with the anti-TNF-alpha antibody infliximab. Rheumatology (Oxford). 2007;46:1450–3.
Braun J, Baraliakos X, Listing J, et al. Persistent clinical efficacy and safety of anti-tumour necrosis factor alpha therapy with infliximab in patients with ankylosing spondylitis over 5 years: evidence for different types of response. Ann Rheum Dis. 2008;67(3):340–5.
Wanders A, Dv Heijde, Landewé R, et al. Non steroidal anti-inflammatory drugs induce radiographic progression in patients with ankylosing spondylitis: a randomized clinic trial. Arthritis Rheum. 2005;52:1756–65.
Boersma JW. Retardation of ossification on the lumbar vertebral column in ankylosing spondylitis by means of phenylbutazone. Scan J Rheum. 1976;5:60–4.
Krischak GD, Augat P, Blakytny R, Claes L, Kinzl L, Beck A. The non steroidal anti inflammatory drug diclofenac reduces appearance of osteoblasts in bone defect healing in rats. Arch Orthop Trauma Surg. 2007;127:453–8.
Qian J, Xie J, Hong S, et al. Dickkopf-1 (DKK1) is a widely expressed and potent tumor associated antigen in multiple myeloma. Blood 2007;110:1587–1594.
Claudepierre P, Wendling D. Are inflammation and ossification on separate tracks in ankylosing spondylitis? Joint Bone Spine. 2008;75:520–2.
François RJ, Neure L, Sieper J, Braun J. Immunohistological examination of open sacroiliac biopsies of patients with ankylosing spondylitis: detection of tumor necrosis factor alpha in two patients with early disease and transforming growth factor beta in three more advanced cases. Ann Rheum Dis. 2006;65:713–20.
Braun J, Bollow M, Neure L, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arth Rheum. 1995;38:499–505.
Archer JR. Ankylosing spondylitis, IgA, and transforming growth factors. Ann Rheum Dis. 1995;54:544–6.
Claudepierre P, Rymer JC, Authier FJ, et al. A relationship between TGF-beta 1 or IL-6 plasma levels and clinical features of spondyloarthropathies. Br J Rheumatol. 1997;36:400–1.
Wendling D, Cedoz JP, Racadot E, Dumoulin G. Serum IL17, BMP-7, and bone turnover markers in patients with ankylosing spondylitis. Joint Bone Spine. 2007;74:304–5.
Roux S, Orcel P. Bone loss. Factors that regulate osteoclast differentiation: un up-date. Arthritis Res. 2000;2:451–6.
Li P, Schwarz EM, O’Keefe RJ, et al. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11b high osteoclast precursor in tumor necrosis factor alpha-transgenic mice. Arthritis Rheum. 2004;50:265–76.
Kaneki H, Guo R, Chen D, et al. Tumor necrosis factor promotes Runx2 degradation through up-regulation of Smurf1 and Smurf2 in osteoblasts. J Biol Chem. 2006;281:4326–33.
Ji H, Pettit A, Ohmura K, et al. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induces arthritis. J Exp Med. 2002;196:77–85.
Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282–90.
Zwerina J, Redlich K, Polzer K, et al. TNF-induced structural joint damage is mediated by IL-1. Proc Natl Acad Sci USA. 2007;104:11742–7.
Fonseca JE, Santos MJ, Canhão H, Choy E. IL6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev. 2009;8:538–42.
Kato A, Matsuo S, Takai H, Uchiyama Y, Mihara M, Suzuki M. Early effects of tocilizumab on bone and bone marrow lesions in a collagen-induced arthritis monkey model. Exp Mol Pathol. 2008;84:262–70.
Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69(1):88–96.
Ogura H, Murakami M, Okuyama Y, et al. IL17 promotes autoimmunity by triggering a positive feed-back loop via IL6 induction. Immunity. 2008;29:628–36.
Poole KE, van Bezooijen RL, Loveridge N, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–4.
Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–15.
Walsh NC, Reinwald S, Manning CA, et al. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res. 2009;24:1572–85.
Bodine PV, Komm BS. Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:33–9.
Moon RT, Kohn AD, De Ferrari GV, Kaykas A. Wnt and beta-catenin signaling: diseases and therapies. Nat Rev Genet. 2004;5:691–701.
Ijiri K, Nagayoshi R, Matsushita N, et al. Differential expression patterns of secreted frizzled related protein genes in synovial cells from patients with arthritis. J Rheumatol. 2002;29:2266–70.
Arnett TR, Gibbons DC, Utting JC, et al. Hypoxia is a major regulator of osteoclast formation and bone resorption. J Cell Physiol. 2003;196:2–8.
Utting JC, Robins SP, Brandao-Burch A, Orriss IR, Behar J, Arnett TR. Hypoxia inhibits the growth, differentiation and bone forming capacity of rat osteoblasts. Exp Cell Res. 2006;312:1693–702.
Brandao-Burch A, Utting JC, Orriss IR, Arnett TR. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif Tissue Int. 2005;77:167–74.
Uderhardt S, Diarra D, Katzenbeisser J, et al. Blockade of Dickkopf 1 induces fusion of sacroiliac joints. Ann Rheum Dis. 2010;69(3):592–7.
Li P, Schwarz EM, O’Keefe RJ, Ma L, Boyce BF, Xing L. RANK signaling is not required for TNFalpha-mediated increase in CD11(hi) osteoclasts precursor but is essential for mature osteoclast formation in TNFalpha-mediated inflammatory arthritis. J Bone Miner Res. 2004;19:207–13.
Redlich K, Hayer S, Maier A, et al. Tumor necrosis factor alpha-mediated joint destruction is inhibited by targeting osteoclast with osteoprotegerin. Arthritis Rheum. 2002;46:785–92.
Cohen SB, Dore RK, Lane NE, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58:1299–309.
Lencel P, Delplace S, Pilet P, et al. Cell-specific effects of TNF-alpha and IL-1 beta on alkaline phosphatase: implication for syndesmophyte formation and vascular calcification. Lab Invest. 2001;91:1434–42.
Pham T. Pathophysiology of ankylosing spondylitis: what’s new? Joint Bone Spine. 2008;75:656–60.
Brown MA, Kennedy LG, MacGregor AJ, et al. Susceptibility to ankylosing spondylitis in twins: the role of genes, HLA, and the environment. Arthritis Rheum. 1997;40:1823–8.
D’Amato M, Fiorillo MT, Carcassi C, et al. Relevance of residue 116 of HLA-B27 in determining susceptibility to ankylosing spondylitis. Eur J Immunol. 1995;25:3199–201.
Ramos M, López de Castro JA. HLA-B27 and the pathogenesis of spondyloarthritis. Tissue Antigens. 2002;60:191–205.
Cauli A, Vacca A, Mameli A, et al. A Sardinian patient with ankylosing spondylitis and HLA-B*2709 co-occurring with HLA-B*1403. Arthritis Rheum. 2007;56:2807–9.
Olivieri I, D’Angelo S, Scarano E, Santospirito V, Padula A. The HLA-B*2709 subtype in a woman with early ankylosing spondylitis. Arthritis Rheum. 2007;56:2805–7.
Fiorillo MT, Cauli A, Carcassi C, et al. Two distinctive HLA haplotypes harbor the B27 alleles negatively or positively associated with ankylosing spondylitis in Sardinia: implications for disease pathogenesis. Arthritis Rheum. 2003;48:1385–9.
Cascino I, Paladini F, Belfiore F, et al. Identification of previously unrecognized predisposing factors for ankylosing spondylitis from analysis of HLA-B27 extended haplotypes in Sardinia. Arthritis Rheum. 2007;56:2640–51.
López-Larrea C, Sujirachato K, Mehra NK, et al. HLA-B27 subtypes in Asian patients with ankylosing spondylitis. Evidence for new associations. Tissue antigens. 1995;45:169–76.
Gonzalez-Roces S, Alvarez MV, Gonzalez S, et al. HLA-B27 polymorphism and worldwide susceptibility to ankylosing spondylitis. Tissue Antigens. 1997;49:116–23.
Varnavidou-Nicolaidou A, Karpasitou K, Georgiou D, et al. HLA-B27 in the Greek Cypriot population: distribution of subtypes in patients with ankylosing spondylitis and other HLA-B27-related diseases. The possible protective role of B*2707. Human Immunol. 2004;65:1451–4.
Sprent J, Schaefer M. Antigen presenting cells for CD8+ cells. Immunol Rev. 1990;117:213–34.
Taurog JD, Maika SD, Simmons WA, Breban M, Hammer RE. Susceptibility to inflammatory disease in HLA-B27 transgenic rat lines correlates with the level of B27 expression. J Immunol. 1993;150:4168–78.
Cauli A, Dessole G, Passiu G, Mathieu A. Quantification of cellular antigens by means of flow cytometry and its role in rheumatology. Reumatismo. 2001;53:14–7.
Poncelet P. Microbeads and flow cytometry: how and why put the “-metry” in immuno-cytometry? Ann Biol Clin (Paris). 2004;62(1):53–7.
Cauli A, Dessole G, Nurchis PP, et al. The role of HLA-B27 molecules in the pathogenesis of ankylosing spondylitis. Reumatismo. 2002;54(3):266–71.
Cauli A, Dessole G, Fiorillo MT, et al. Increased level of HLA-B27 expression in ankylosing spondylitis patients compared with healthy HLA-B27 positive subjects: a possible further susceptibility factor for the development of disease. Rheumatology (Oxford). 2002;41(12):1375–9.
Ebringer A, Ahmadi K, Fielder M, et al. Molecular mimicry: the geographical distribution of immune responses to Klebsiella in ankylosing spondylitis and its relevance to therapy. Clin Rheumatol. 1996;15:57–67.
Tsuchiya N, Husby G, Williams RC Jr, Stieglitz H, Lipsky PE, Inman RD. Autoantibodies to HLA-B27 sequence cross-react with the hypothetical peptide from the arthritis—associated Shigella plasmid. J Clin Invest. 1990;85:1193–203.
Fiorillo MT, Maragno M, Butler R, Dupuis ML, Sorrentino R. CD8+ T cell auto-reactivity to HLA-B27-restricted self-epitope correlates with ankylosing spondylitis. J Clin Invest. 2000;106:47–53.
Mear JP, Schreiber KL, Münz C, et al. Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J Immunol. 1999;163:6665–70.
Lipsky PE. Spondyloarthopathies. In: Klippel JH, Dieppe PA, editors. Rheumatology 2nd ed. London: Mosby, 1999: 6.12.1–12.
Khare SD, Hansen J, Luthra HS, David CS. HLA-B27 heavy chains contribute to spontaneous inflammatory disease in B27/human beta 2-microglobulin (beta2 m) double transgenic mice with disrupted mouse beta2m. J Clin Invest. 1996;98:2746–55.
Allen RL, O’Callaghan CA, McMichael AJ, Bowness P. Cutting edge: HLA-B27 can form a novel beta 2-microglobulin-free heavy chain homodimer structure. J immunol. 1999;162:5045–8.
Kim TH, Stone MA, Rahman P, et al. Interleukin 1 and nuclear factor-kappaB polymorphism in ankylosing spondylitis in Canada and Korea. J Rheumatol. 2005;32:1907–10.
Sims AM, Timms AE, Bruges-Armas J, et al. Prospective meta-analysis of interleukin 1 gene complex polymorphisms confirm associations with ankylosing spondylitis. Ann Rheum Dis. 2008;67:1305–9.
Maksymowych WP, Rahman P, Reeve JP, Gladman DD, Peddle L, Inman RD. Association of the IL1 gene cluster with susceptibility to ankylosing spondylitis: an analysis of three Canadian populations. Arthritis Rheum. 2006;54:974–85.
Niki Y, Yamada H, Kikuchi T, et al. Membrane associated IL-1 contributes to chronic synovitis and cartilage destruction in human IL-1 alpha transegenic mice. J Immunol. 2004;172:577–84.
Tan AL, Marzo-Ortega H, O’Connor P, Fraser A, Emery P, McGonagle D. Efficacy of anakinra in active ankylosing spondylitis: a clinical and magnetic resonance imaging study. Ann Rheum Dis. 2004;63:1041–5.
Haibel H, Rudwaleit M, Listing J, Sieper J. Open label trial of anakinra in active ankylosing spondylitis over 24 weeks. Ann Rheum Dis. 2005;64:296–8.
Yan J, Parekh VV, Mendez-Fernandez Y, et al. In vivo role of ER-associated peptidase activity in tailoring peptides for presentation by MHC class Ia and class Ib molecules. J Exp Med. 2006;203:647–59.
Hammer ND, Schmidt JC, Chapman MR. The curli nucleator protein, CsgB, contains an amyloidogenic domain that directs CsgA polymerization. Proc Natl Acad Sci USA. 2007;104:12494–9.
McGovern D, Powrie F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut. 2007;56:1333–6.
Wendling D. Interleukin 23: a key cytokine in chronic inflammatory disease. Joint Bone Spine. 2008;75:517–9.
Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukin 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–9.
Zheng Y, Valdez PA, Danilenko DM, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–9.
Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–92.
Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial. (PHOENIX 2). Lancet. 2008;371:1675–84.
Goldminz AM, Gottlieb AB. Ustekinumab for psoriasis and psoriatic arthritis. J Rheumatol Suppl. 2012;89:86–9. doi:10.3899/jrheum.120253.
Rudwaleit M, Siegert S, Yin Z, et al. Low T cell production of TNF alpha and INF gamma in ankylosing spondylitis: its relation to HLA-B27 and influence of the TNF-308 gene polymorphism. Ann Rheum Dis. 2001;60:36–42.
Smith JA, Barnes MD, Hong D, DeLay ML, Inman RD, Colbert RA. Gene expression analysis of macrophages derived from ankylosing spondylitis patients reveals interferon-gamma dysregulation. Arthritis Rheum. 2008;58(6):1640–9.
Layh-Schmitt G, Colbert RA. The interleukin-23/interleukin-17 axis in spondyloarthritis. Curr Opin Rheumatol. 2008;20:392–7.
van der Heijde D, Machado P, Braun J, et al. MRI inflammation at the vertebral unit only marginally predicts new syndesmophyte formation: a multilevel analysis in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71(3):369–73.
Schett G, Rudwaleit M. Can we stop progression of ankylosing spondylitis ? Best Pract Res Clin Rheumatol. 2010;24(3):363–71.
Sieper J, Appel H, Braun J, Rudwaleit M. Critical appraisal of assessment of structural damage in ankylosing spondylitis: implications for treatment outcomes. Arthritis Rheum. 2008;58(3):649–56.
Hermann KG, Baraliakos X, van der Heijde DM, et al. Descriptions of spinal MRI lesions and definition of a positive MRI of the spine in axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI study group. Ann Rheum Dis. 2012;71(8):1278–88.
Chiowchanwisawakit P, Lambert RG, Conner-Spady B, Maksymowych WP. Focal fat lesions at vertebral corners on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis. Arthritis Rheum. 2011;63(8):2215–25.
Maksymowych WP, Chiowchanwisawakit P, Clare T, Pedersen SJ, Østergaard M, Lambert RG. Inflammatory lesions of the spine on magnetic resonance predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum. 2009;60(1):93–102.
Acknowledgments
We apologize for not citing many important references that have contributed to this review due to space limitations. The authors are grateful for support to their research from the “5 × 1000” voluntary contribution. None of the authors received any funding related to the writing of this manuscript, and the funding bodies did not play any role in the writing of the manuscript or decision to submit the manuscript for publication.
Conflict of interest
The authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Cata, A., Inglese, M., Rubino, R. et al. The synovio-entheseal complex in enthesoarthritis. Clin Exp Med 16, 109–124 (2016). https://doi.org/10.1007/s10238-015-0341-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-015-0341-x