Abstract
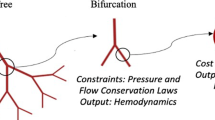
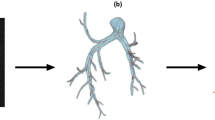
Hemodynamic loading is known to contribute to the development and progression of pulmonary arterial hypertension (PAH). This loading drives changes in mechanobiological stimuli that affect cellular phenotypes and lead to pulmonary vascular remodeling. Computational models have been used to simulate mechanobiological metrics of interest, such as wall shear stress, at single time points for PAH patients. However, there is a need for new approaches that simulate disease evolution to allow for prediction of long-term outcomes. In this work, we develop a framework that models the pulmonary arterial tree through adaptive and maladaptive responses to mechanical and biological perturbations. We coupled a constrained mixture theory-based growth and remodeling framework for the vessel wall with a morphometric tree representation of the pulmonary arterial vasculature. We show that non-uniform mechanical behavior is important to establish the homeostatic state of the pulmonary arterial tree, and that hemodynamic feedback is essential for simulating disease time courses. We also employed a series of maladaptive constitutive models, such as smooth muscle hyperproliferation and stiffening, to identify critical contributors to development of PAH phenotypes. Together, these simulations demonstrate an important step toward predicting changes in metrics of clinical interest for PAH patients and simulating potential treatment approaches.









Similar content being viewed by others
Data availability
All data and code from this work are available on request.
References
Abman SH, Hansmann G, Archer SL et al (2015) Pediatric pulmonary hypertension: guidelines from the American heart association and American thoracic society. Circulation 132(21):2037–2099
Acosta S, Puelz C, Rivière B et al (2017) Cardiovascular mechanics in the early stages of pulmonary hypertension: a computational study. Biomech Model Mechanobiol 16:2093–2112
Bartolo MA, Qureshi MU, Colebank MJ et al (2022) Numerical predictions of shear stress and cyclic stretch in pulmonary hypertension due to left heart failure. Biomech Model Mechanobiol 21(1):363–381
Chambers MJ, Colebank MJ, Qureshi MU et al (2020) Structural and hemodynamic properties of murine pulmonary arterial networks under hypoxia-induced pulmonary hypertension. Proc Inst Mech Eng Part H J Eng Med 234(11):1312–1329
Champion HC, Villnave DJ, Tower A et al (2000) A novel right-heart catheterization technique for in vivo measurement of vascular responses in lungs of intact mice. Am J Physiol Heart Circ Physiol 278(1):H8–H15
Chan XY, Volkova E, Eoh J et al (2021) Hif2a gain-of-function mutation modulates the stiffness of smooth muscle cells and compromises vascular mechanics. IScience 24(4):102–246
Cocciolone AJ, Hawes JZ, Staiculescu MC et al (2018) Elastin, arterial mechanics, and cardiovascular disease. Am J Physiol Heart Circ Physiol 315(2):H189–H205
Deng H, Min E, Baeyens N et al (2021) Activation of smad2/3 signaling by low fluid shear stress mediates artery inward remodeling. Proc Nat Acad Sci 118(37):e2105339
Dong M, Yang W, Tamaresis JS et al (2020) Image-based scaling laws for somatic growth and pulmonary artery morphometry from infancy to adulthood. Am Physiol Heart Circ Physiol 319(2):H432–H442
Dong ML, Lan IS, Yang W et al (2021) Computational simulation-derived hemodynamic and biomechanical properties of the pulmonary arterial tree early in the course of ventricular septal defects. Biomechan Model Mechanobiol 20(6):2471–2489
Gharahi H, Filonova V, Mullagura HN et al (2023) A multiscale framework for defining homeostasis in distal vascular trees: applications to the pulmonary circulation. Biomech Model Mechanobiol 22(3):971–86
Ghorishi Z, Milstein JM, Poulain FR et al (2007) Shear stress paradigm for perinatal fractal arterial network remodeling in lambs with pulmonary hypertension and increased pulmonary blood flow. Am J Physiol Heart Circ Physiol 292(6):H3006–H3018
Guo J, Yang ZC, Liu Y (2019) Attenuating pulmonary hypertension by protecting the integrity of glycocalyx in rats model of pulmonary artery hypertension. Inflammation 42:1951–1956
Hislop A, Reid L (1973) Pulmonary arterial development during childhood: branching pattern and structure. Thorax 28(2):129–135
Hislop A, Reid L (1978) Normal structure and dimensions of the pulmonary arteries in the rat. J Anat 125(Pt 1):71
Huang W, Yen R, McLaurine M et al (1996) Morphometry of the human pulmonary vasculature. J Appl Physiol 81(5):2123–2133
Humbert M, Montani D, Perros F et al (2008) Endothelial cell dysfunction and cross talk between endothelium and smooth muscle cells in pulmonary arterial hypertension. Vasc Pharmacol 49(4–6):113–118
Humphrey J (2021) Constrained mixture models of soft tissue growth and remodeling-twenty years after. J Elast 145(1):49–75
Humphrey JD, Schwartz MA (2021) Vascular mechanobiology: homeostasis, adaptation, and disease. Ann Rev Biomed Eng 23:1–27
Ivy D (2016) Pulmonary hypertension in children. Cardiol clin 34(3):451–472
Jiang Z, Kassab G, Fung Y (1994) Diameter-defined strahler system and connectivity matrix of the pulmonary arterial tree. J Appl Physiol 76(2):882–892
Kameny RJ, Datar SA, Boehme JB et al (2019) Ovine models of congenital heart disease and the consequences of hemodynamic alterations for pulmonary artery remodeling. Am J Respir Cell Mol Biol 60(5):503–514
Kheyfets VO, Rios L, Smith T et al (2015) Patient-specific computational modeling of blood flow in the pulmonary arterial circulation. Comput Methods Prog Biomed 120(2):88–101
Kim YM, Haghighat L, Spiekerkoetter E et al (2011) Neutrophil elastase is produced by pulmonary artery smooth muscle cells and is linked to neointimal lesions. Am J Pathol 179(3):1560–1572
Lan IS, Yang W, Feinstein JA et al (2022) Virtual transcatheter interventions for peripheral pulmonary artery stenosis in williams and alagille syndromes. J Am Heart Assoc 11(6):e023532
Latorre M, Humphrey JD (2020) Numerical knockouts-in silico assessment of factors predisposing to thoracic aortic aneurysms. PLoS Comput Biol 16(10):e1008273
Latorre M, Szafron JM, Ramachandra AB et al (2022) In vivo development of tissue engineered vascular grafts: a fluid-solid-growth model. Biomech Model Mechanobiol 21(3):827–848
Leach R, Twort C, Cameron I et al (1979) A comparison of the pharmacological and mechanical properties in vitro of large and small pulmonary arteries of the rat. Clin Sci 82(1):55–62
Lechartier B, Berrebeh N, Huertas A et al (2022) Phenotypic diversity of vascular smooth muscle cells in pulmonary arterial hypertension: implications for therapy. Chest 161(1):219–231
Lee P, Carlson BE, Chesler N et al (2016) Heterogeneous mechanics of the mouse pulmonary arterial network. Biomech Model Mechanobiol 15(5):1245–1261
Li G, Wang M, Caulk AW et al (2020) Chronic mtor activation induces a degradative smooth muscle cell phenotype. J Clin Investig 130(3):1233–1251
Marsden AL (2013) Simulation based planning of surgical interventions in pediatric cardiology. Phys Fluids 25(10):101303
Moonen JR, Lee ES, Schmidt M et al (2015) Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovasc Res 108(3):377–386
Moonen JR, Chappell J, Shi M et al (2022) Klf4 recruits swi/snf to increase chromatin accessibility and reprogram the endothelial enhancer landscape under laminar shear stress. Nat Communi 13(1):1–16
Phillips MR, Moore SM, Shah M et al (2017) A method for evaluating the murine pulmonary vasculature using micro-computed tomography. J Surg Res 207:115–122
Postles A, Clark AR, Tawhai MH (2014) Dynamic blood flow and wall shear stress in pulmonary hypertensive disease. In: 2014 36th annual international conference of the IEEE engineering in medicine and biology society, IEEE, pp 5671–5674
Prapa M, McCarthy KP, Dimopoulos K et al (2013) Histopathology of the great vessels in patients with pulmonary arterial hypertension in association with congenital heart disease: large pulmonary arteries matter too. Int J Cardiol 168(3):2248–2254
Pries A, Secomb T, Gaehtgens P (1998) Structural adaptation and stability of microvascular networks: theory and simulations. Am J Physiol Heart Circ Physiol 275(2):H349–H360
Pries A, Reglin B, Secomb T (2001) Structural adaptation of microvascular networks: functional roles of adaptive responses. Am J Physiol Heart Circ Physiol 281(3):H1015–H1025
Qureshi MU, Vaughan GD, Sainsbury C et al (2014) Numerical simulation of blood flow and pressure drop in the pulmonary arterial and venous circulation. Biomech Model Mechanobiol 13(5):1137–1154
Rabinovitch M, Guignabert C, Humbert M et al (2014) Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 115(1):165–175
Rachev A, Hayashi K (1999) Theoretical study of the effects of vascular smooth muscle contraction on strain and stress distributions in arteries. Ann Biomed Eng 27(4):459–468
Ramachandra AB, Humphrey JD (2019) Biomechanical characterization of murine pulmonary arteries. J Biomech 84:18–26
Razavi H, Dusch MN, Zarafshar SY et al (2012) A method for quantitative characterization of growth in the 3-d structure of rat pulmonary arteries. Microvasc. Res 83(2):146–153
Satoh K, Satoh T, Kikuchi N et al (2014) Basigin mediates pulmonary hypertension by promoting inflammation and vascular smooth muscle cell proliferation. Circ Res 115(8):738–750
Secomb TW (2017) Blood flow in the microcirculation. Ann Rev Fluid Mech 49:443–461
Spronck B, Latorre M, Wang M et al (2021) Excessive adventitial stress drives inflammation-mediated fibrosis in hypertensive aortic remodelling in mice. J R Soc Interface 18(180):20210336
Szafron J, Khosravi R, Reinhardt J et al (2018) Immuno-driven and mechano-mediated neotissue formation in tissue engineered vascular grafts. Ann Biomed Eng 46(11):1938–1950
Tang BT, Pickard SS, Chan FP et al (2012) Wall shear stress is decreased in the pulmonary arteries of patients with pulmonary arterial hypertension: an image-based, computational fluid dynamics study. Pulm Circ 2(4):470–476
Tojais NF, Cao A, Lai YJ et al (2017) Codependence of bone morphogenetic protein receptor 2 and transforming growth factor-\(\beta\) in elastic fiber assembly and its perturbation in pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 37(8):1559–1569
Townsley MI (2012) Structure and composition of pulmonary arteries, capillaries and veins. Compr Physiol 2:675
Valentin A, Cardamone L, Baek S et al (2009) Complementary vasoactivity and matrix remodelling in arterial adaptations to altered flow and pressure. J R Soc Interface 6(32):293–306
Yang TL, Lee PL, Lee DY et al (2018) Differential regulations of fibronectin and laminin in smad2 activation in vascular endothelial cells in response to disturbed flow. J Biomed Sci 25(1):1–13
Yang W, Feinstein JA, Vignon-Clementel IE (2016) Adaptive outflow boundary conditions improve post-operative predictions after repair of peripheral pulmonary artery stenosis. Biomech Model Mechanobiol 15:1345–1353
Yang W, Dong M, Rabinovitch M et al (2019) Evolution of hemodynamic forces in the pulmonary tree with progressively worsening pulmonary arterial hypertension in pediatric patients. Biomech Model Mechanobiol 18(3):779–796
Zambrano BA, McLean NA, Zhao X et al (2018) Image-based computational assessment of vascular wall mechanics and hemodynamics in pulmonary arterial hypertension patients. J Biomech 68:84–92
Zhang M, Feng Z, Huang R et al (2018) Characteristics of pulmonary vascular remodeling in a novel model of shunt-associated pulmonary arterial hypertension. Med Sci Monit Int Med J Exp Clini Res 24:1624
Funding
The authors appreciate funding support from the Parker B. Francis Fellowship program for this work. This work was also supported in part by the Stanford Maternal and Child Health Research Institute through the Pilot Award Program and a grant from the National Heart, Lung and Blood Institute (1T32HL098049).
Author information
Authors and Affiliations
Contributions
JMS performed the simulations and data analysis with guidance from WY and ALM. JMS, MR, and ALS conceptualized the study design. JAF and MR provided clinical context for the results and discussion. JMS, WY, and ALS wrote the main manuscript text. All authors reviewed and edited the text.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Ethical approval
This work involved no human or animal experiments requiring ethical approval.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Szafron, J.M., Yang, W., Feinstein, J.A. et al. A computational growth and remodeling framework for adaptive and maladaptive pulmonary arterial hemodynamics. Biomech Model Mechanobiol 22, 1935–1951 (2023). https://doi.org/10.1007/s10237-023-01744-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-023-01744-z