Abstract
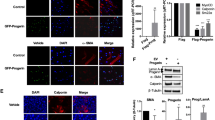
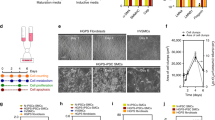
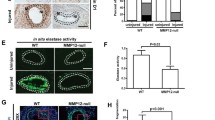
Hutchinson–Gilford Progeria Syndrome results in rapid aging and severe cardiovascular sequelae that accelerate near end-of-life. We found a progressive disease process in proximal elastic arteries that was less evident in distal muscular arteries. Changes in aortic structure and function were then associated with changes in transcriptomics assessed via both bulk and single cell RNA sequencing, which suggested a novel sequence of progressive aortic disease: adverse extracellular matrix remodeling followed by mechanical stress-induced smooth muscle cell death, leading a subset of remnant smooth muscle cells to an osteochondrogenic phenotype that results in an accumulation of proteoglycans that thickens the aortic wall and increases pulse wave velocity, with late calcification exacerbating these effects. Increased central artery pulse wave velocity is known to drive left ventricular diastolic dysfunction, the primary diagnosis in progeria children. It appears that mechanical stresses above ~ 80 kPa initiate this progressive aortic disease process, explaining why elastic lamellar structures that are organized early in development under low wall stresses appear to be nearly normal whereas other medial constituents worsen progressively in adulthood. Mitigating early mechanical stress-driven smooth muscle cell loss/phenotypic modulation promises to have important cardiovascular implications in progeria patients.





Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions of the paper are in the paper and the Supplementary Materials. Additional information is available from the corresponding author upon reasonable request.
References
Bayer IM, Adamson SL, Langille BL (1999) Atrophic remodeling of the artery-cuffed artery. Arterioscler Thromb Vasc Biol 19:1499–1505
Benedicto I, Dorado B, Andres V (2021) Molecular and cellular mechanisms driving cardiovascular disease in Hutchinson-Gilford progeria syndrome: lessons learned from animal models. Cells 10:1157
Bibby JA, Agarwal D, Freiwald T, Kunz N, Merle NS, West EE, Singh P, Larochelle A, Chinian F, Mukherjee S, Afzali B, Kemper C, Zhang NR (2022) Systematic single-cell pathway analysis to characterize early T cell activation. Cell Rep 41:111697
Boers JLV, Peeters EAG, Kuijpers JHJ et al (2004) Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum Mol Genet 13:2567–2580
Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF (2021) Arterial Stiffness and Cardiovascular Risk in Hypertension. Circ Res 128:864–886
Briot A, Jaroszewicz A, Warren CM et al (2014) Repression of Sox9 by Jag1 is continuously required to suppress the default chondrogenic fate of vascular smooth muscle cells. Dev Cell 31:707–721
Capell BC, Olive M, Erdos MR, Cao K, Faddah DA, Tavarez UL, Chen X (2008) A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model. Proc Natl Acad Sci USA 105:15902–15907
Cavinato C, Murtada S-I, Rojas A, Humphrey JD (2021) Evolving structure-function relations during aortic maturation and aging revealed by multiphoton microscopy. Mechan Ageing Devel 196:111471
Chambliss AB, Khatau SB, Erdenberger N, Robinson DK, Hodzic D, Longmore GD, Wirtz D (2013) The LINC-anchored actin cap connects the extracellular milieus to the nucleus for ultrafast mechanotransduction. Sci Rept 3:1087
Clarke MCH, Littlewood TD, Figg N, Maguire JJ, Davenport AP, Goddard M, Bennett MR (2008) Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ Res 102:1529–1538
De Sandre-Giovannoli A, Bernard R, Cau P et al (2003) Lamin A truncation in Hutchinson-Gilford Progeria. Science 300:2055
Del Campo L, Sánchez-López A, Salaices M, von Kleeck RA, Expósito E, González-Gómez C, Cussó L, Guzmán-Martínez G, Ruiz-Cabello J, Desco M, Assoian RK, Briones AM, Andrés V (2019) Vascular smooth muscle cell-specific progerin expression in a mouse model of Hutchinson-Gilford progeria syndrome promotes arterial stiffness: therapeutic effect of dietary nitrite. Aging Cell 18:e12936
Desai AS, Mitchell GF, Fang JC, Creager MA (2009) Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail 15:658–664
Dolgalev I (2022). msigdbr: MSigDB Gene sets for multiple organisms in a tidy data format. R package version 7.5.1, https://CRAN.R-project.org/package=msigdbr
DuBose AJ, Lichtenstein ST, Petrash NM, Erdos MR, Gordon LB, Collins FS (2018) Everolimus rescues multiple cellular defects in laminopathy-patient fibroblasts. Proc Natl Acad Sci USA 115:4206–4211
Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM (2018) Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res 114:590–600
Eriksson M, Brown WT, Gordon LB et al (2003) Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423:293–298
Estrada AC, Irons L, Rego BV, Li G, Tellides G, Humphrey JD (2021) Roles of mTOR in thoracic aortopathy understood by complex intracellular signaling interactions. PLoS Comput Biol 17:e1009683
Ferruzzi J, Madziva D, Caulk AW, Tellides G, Humphrey JD (2018) Compromised mechanical homeostasis in arterial aging and associated cardiovascular consequences. Biomech Model Mechanobiol 17:1281–1295
Gerhard-Herman M, Smoot LB, Wake N, Kieran MW, Kleinman ME, Miller DT, Schwartzman A, Giobbie-Hurder A, Neuberg D, Gordon LB (2012) Mechanisms of premature vascular aging in children with Hutchison-Gilford progeria syndrome. Hypertension 59:92–97
Gordon LB, Kleinman ME, Miller DT et al (2012) Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA 109:16666–16671
Gordon LB, Massaro J, D’Agostino RB et al (2014) Impact of farnesylation inhibitors on survival in Hutchinson-Gilford progeria syndrome. Circulation 130:27–34
Gordon LB, Shappell H, Massaro J, D’Agostino RB, Brazier J, Kleinman ME, Kieran MW (2018) Association of lonafarnib treatment vs no treatment with mortality rates in patients with Hutchison-Gilford progeria syndrome. JAMA 319:1687–1695
Humphrey JD, Eberth JF, Dye WW, Gleason RL (2009) Fundamental role of axial stress in compensatory adaptations by arteries. J Biomech 42:1–8
Humphrey JD, Dufresne ER, Schwartz MA (2014) Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15:802–812
Humphrey JD, Tellides G (2019) Central artery stiffness and thoracic aortopathy. Am J Physiol 316:H169-182
Johnson RC, Leopold JA, Loscalzo J (2006) Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res 99:1044–1059
Kelleher CM, McLean SE, Mecham RP (2004) Vascular extracellular matrix and aortic development. Curr Trends Develop Biol 62:153–188
Kim PH, Luu J, Heizer P, et al. (2018) Disrupting the LINC complex in smooth muscle cells reduces aortic disease in a mouse model of Hutchinson-Gilford progeria syndrome. Sci Transl Med 10:eaat7163
Kim PH, Chen NY, Heizer PJ et al (2021) Nuclear membrane ruptures underlie the vascular pathology in a mouse model of Hutchinson-Gilford progeria syndrome. JCI Insight 6:e151515
Kreienkamp R, Billon C, Bedia-Diaz G, Albert CJ, Toth Z, Butler AA, Gonzalo S (2019) Doubled lifespan and patient-like pathologies in progeria mice fed high-fat diet. Aging Cell 18:e12852
Latorre M, Spronck B, Humphrey JD (2021) Complementary roles of mechanotransduction and inflammation in vascular homeostasis. Proc Math Phys Eng Sci 477:20200622
Laurent S, Boutouyrie P (2015) The structural factor of hypertension: large and small artery alterations. Circ Res 116:1007–1021
Le VP, Cheng JK, Kim J et al (2015) Mechanical factors direct mouse aortic remodeling during early maturation. J R Soc Interface 12:20141350
Lemire JM, Patis C, Gordon LB, Sandy JD, Toole BPM, Weiss AS, (2006) Aggrecan expression is substantially and abnormally upregulated in Hutchinson-Gilford progeria syndrome dermal fibroblasts. Mech Ageing Develop 127:660–669
Li G, Wang M, Caulk AW, Korneva A, Bersi MR, Wang G, Liu X, Mehta S, Geirsson A, Gulcher JR, Chittenden TW, Simons M, Humphrey JD, Tellides G (2020a) Chronic mTOR activation induces a degradative smooth muscle cell phenotype and aortopathy. J Clin Invest 130:1233–1251
Li Y, Sun Z, Zhang L, Yan J, Shao C, Jin L, Li L, Wang Z (2020b) Role of macrophages in the progression and regression of vascular calcification. Front Pharmacol 11:661
Liu Y, Drozdov I, Shroff R, Beltran LE, Shanahan CM (2013) Prelamin A accelerates vascular calcification via activation of the DNA damage response and senescence-associated secretory phenotype in vascular smooth muscle cells. Circ Res 112:e99-109
Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P (2015) The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst 1(6):417–425
Michel J-B (2003) Anoikis in the cardiovascular system: known and unknown extracellular mediators. Arterioscler Thromb Vasc Biol 23:2146–2154
Murtada S-I, Kawamura Y, Caulk AW, Ahmadzadeh H, Mikush N, Zimmerman K, Kavanagh D, Weiss D, Latorre M, Zhuang ZW, Shadel GS, Braddock DT, Humphrey JD (2020) Paradoxical aortic stiffening and subsequent cardiac dysfunction in Hutchison-Gilford progeria. J R Soc Interface 17:20200066
Murtada S-I, Kawamura Y, Li G, Schwartz MA, Tellides G, Humphrey JD (2021a) Developmental origins of mechanical homeostasis in the aorta. Dev Dyn 250:629–639
Murtada S-I, Kawamura Y, Humphrey JD (2021b) Differential biomechanical responses of elastic and muscular arteries to angiotensin II-induced hypertension. J Biomech 119:110297
Murtada S-I, Mikush N, Wang M, Ren P, Kawamura Y, Ramachandra AB, Braddock DT, Tellides G, Gordon LB, Humphrey JD (2023) Lonafarnib improves cardiovascular function and survival in a mouse model of Hutchinson-Gilford Progeria Syndrome. eLife 12:e82728.
Ngai D, Lino M, Bendeck MP (2018) Cell-matrix interactions and mataricine signaling in the pathogenesis of vascular calcification. Front Cardiovasc Med 5:174
Olive M, Harten I, Mitchell R et al (2010) Cardiovascular pathology in Hutchinson-Gilford Progeria: correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol 30:2301–2309
Osmanagic-Myers S, Kiss A, Manakanatas C, Hamza O, Sedlmayer F, Szabo PL, Fischer I, Pichtinger P, Podesser BK, Eriksson M, Foisner R (2019) Endothelial progerin expression causes cardiovascular pathology through an impaired mechanoresponse. J Clin Invest 129:531–545
Osorio FG, Navarro CL, Cadinanos J, et al (2011) Splicing-directed therapy in a new mouse model of human accelerated aging. Sci Transl Med 3: 106ra107
Prakash A, Gordon LB, Kleinman ME, Gurary EB, Massaro J, D’Agostino R, Kieran MW, Gerhard-Herman M, Smoot L (2018) Cardiac abnormalities in patients with Hutchison-Gilford progeria syndrome. JAMA Cardiol 3:326–334
Purnomo E, Emoto N, Nugrahaningshi DAA et al (2013) Glycosaminoglycan overproduction in the aorta increases aortic calcification in murine chronic kidney disease. J Am Heart Assoc 2:e000405
Roccabianca S, Bellini C, Humphrey JD (2014) Computational modeling suggests good, bad, and ugly roles of glycosaminoglycans in arterial mechanics and mechanobiology. J R Soc Interface 11:20140397
Sage AP, Tintut Y, Demer LL (2010) Regulatory mechanisms in vascular calcification. Nat Rev Cardiol 7:528–536
Sanchez-Lopez A, Espinos-Estevez C, Gonzalez-Gomez C, et al. (2021) Cardiovascular progerin suppression and lamin A restoration rescues Hutchinson-Gilford progeria syndrome. Circulation 144:1777–1794
Sazonova OV, Isenberg BC, Herrmann J et al (2015) Extracellular matrix presentation modulates vascular smooth muscle cell mechanotransduction. Matrix Biol 41:36–43
Schroeder F, Polzer S, Slazansky M, Man V, Skacel P (2018) Predictive capabilities of various constitutive models for arterial tissue. J Mech Behav Biomed Matl 78:369–380
Stehbens WE, Wakefield SJ, Gilbert-Barness E, Olson RE, Ackerman J (1999) Histological and ultrastructural features of atherosclerosis in progeria. Cardiovasc Pathol 8:29–39
Stehbens WE, Delahunt B, Shozawa T, Gilbert-Barness E (2001) Smooth muscle cell depletion and collagen types in progeric arteries. Cardiovasc Path 10:133–136
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102(43):15545–15550
Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PDP, Pinter J, Rehfeldt F (2013) Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341:1240104
Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL (2002) Monocyte/macrophage regulation of vascular calcification in vitro. Circulation 105:650–655
Townsend RR, Wilkinson IB, Schiffrin EL, et al. on behalf of the American Heart Association Council on Hypertension (2015) Recommendations for improving and standardizing vascular research on arterial stiffness. Hypertension 66:698–722
Varga R, Eriksson M, Erdos MR et al (2006) Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA 103:3250–3255
Verstraeten VLRM, Ji JY, Cummings KS, Lee RT, Lammerding J (2008) Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: effects of farnesyltransferase inhibitors. Aging Cell 7:383–393
Villa-Bellosta R, Rivera-Torres J, Osorio FG, Acin-Perez R, Enriquez JA, Lopez-Otin C, Andres V (2013) Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson-Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation 127:3442–3451
Acknowledgements
This work was supported by a grant from the US NIH (R01 HL105297, JDH) and the Masason Foundation (YK).
Author information
Authors and Affiliations
Contributions
Design of the project was contributed by SIM, YK, GT, JDH; Data collection was contributed by SIM, YK, CC, MW, ABR, BS; Data analysis and interpretation were contributed by SIM, YK, CC, DSL, GT, JDH. Writing and editing were contributed by SIM, YK, CC, GT, JDH.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
All live animal procedures were conducted in accordance with Federal Regulations and were approved by the Yale Institutional Animal Care and Use Committee prior to beginning the work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Murtada, SI., Kawamura, Y., Cavinato, C. et al. Biomechanical and transcriptional evidence that smooth muscle cell death drives an osteochondrogenic phenotype and severe proximal vascular disease in progeria. Biomech Model Mechanobiol 22, 1333–1347 (2023). https://doi.org/10.1007/s10237-023-01722-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-023-01722-5