Abstract
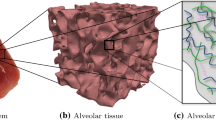
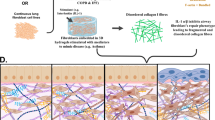
The extracellular matrix (ECM) comprises a large proportion of the lung parenchymal tissue and is an important contributor to the mechanical properties of the lung. The lung tissue is a biologically active scaffold with a complex ECM matrix structure and composition that provides physical support to the surrounding cells. Nearly all respiratory pathologies result in changes in the structure and composition of the ECM; however, the impact of these alterations on the mechanical properties of the tissue is not well understood. In this study, a novel network model was developed to incorporate the combinatorial effect of lung tissue ECM constituents such as collagen, elastin and proteoglycans (PGs) and used to mimic the experimentally derived length–tension response of the tissue to uniaxial loading. By modelling the effect of collagen elasticity as an exponential function with strain, and in concert with the linear elastic response of elastin, the network model’s mechanical response matched experimental stress–strain curves from the literature. In addition, by incorporating spring–dashpot viscoelastic elements, to represent the PGs, the hysteresis response was also simulated. Finally, by selectively reducing volume fractions of the different ECM constituents, we were able to gain insight into their relative mechanical contribution to the larger scale tissue mechanical response.







Similar content being viewed by others
References
Al Jamal R, Roughley PJ, Ludwig MS (2001) Effect of glycosaminoglycan degradation on lung tissue viscoelasticity. Am J Physiol Lung Cellul Mol Physiol 280:L306–L315
Andrikakou P, Vickraman K, Arora H (2016) On the behaviour of lung tissue under tension and compression. Sci Rep 6:36642. https://doi.org/10.1038/srep36642
Annoni R et al (2012) Extracellular matrix composition in COPD. Eur Respir J 40:1362–1373
Asgari M, Latifi N, Heris HK, Vali H, Mongeau L (2017) In vitro fibrillogenesis of tropocollagen type III in collagen type I affects its relative fibrillar topology and mechanics. Sci Rep 7:1392. https://doi.org/10.1038/s41598-017-01476-y
Bates JHT, Maksym GN, Navajas D, Suki B (1994) Lung tissue rheology and 1/f noise. Ann Biomed Eng 22:674–681. https://doi.org/10.1007/bf02368292
Bates JH, Davis GS, Majumdar A, Butnor KJ, Suki B (2007) Linking parenchymal disease progression to changes in lung mechanical function by percolation. Am J Respir Crit Care Med 176:617–623
Brewer KK et al (2003) Lung and alveolar wall elastic and hysteretic behavior in rats: effects of in vivo elastase treatment. J Appl Physiol 95:1926–1936
Burgess JK, Mauad T, Tjin G, Karlsson JC, Westergren-Thorsson G (2016) The extracellular matrix–the under-recognized element in lung disease? J Pathol 240:397–409
Burgstaller G, Oehrle B, Gerckens M, White ES, Schiller HB, Eickelberg O (2017) The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J 50:1601805
Cavalcante FS et al (2005) Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J Appl Physiol 98:672–679. https://doi.org/10.1152/japplphysiol.00619.2004
de Ryk J, Thiesse J, Namati E, McLennan G (2007) Stress distribution in a three dimensional, geometric alveolar sac under normal and emphysematous conditions. Int J Chronic Obstr Pulm Dis 2:81
Denny E, Schroter R (2000) Viscoelastic behavior of a lung alveolar duct model. J Biomech Eng 122:143–151
Fung Y (1967) Elasticity of soft tissues in simple elongation. Am J Physiol Leg Content 213:1532–1544
Gao J, Huang W, Yen R (2006) Mechanical properties of human lung parenchyma. Biomed Sci Instrum 42:172–180
Hoppin F Jr, Lee G, Dawson S (1975) Properties of lung parenchyma in distortion. J Appl Physiol 39:742–751
Ito S, Bartolák-Suki E, Shipley JM, Parameswaran H, Majumdar A, Suki B (2006) Early emphysema in the tight skin and pallid mice: roles of microfibril-associated glycoproteins, collagen, and mechanical forces. Am J Respir Cell Mol Biol 34:688–694
Kimmel E, Budiansky B (1990) Surface tension and the dodecahedron model for lung elasticity. J Biomech Eng 112:160–167. https://doi.org/10.1115/1.2891167
Kononov S, Brewer K, Sakai H, Cavalcante FS, Sabayanagam CR, Ingenito EP, Suki B (2001) Roles of mechanical forces and collagen failure in the development of elastase-induced emphysema. Am J Respir Crit Care Med 164:1920–1926
Kowe R, Schroter RC, Matthews FL, Hitchings D (1986) Analysis of elastic and surface tension effects in the lung alveolus using finite element methods. J Biomech 19:541–549. https://doi.org/10.1016/0021-9290(86)90127-2
Licup AJ et al (2015) Stress controls the mechanics of collagen networks. Proc Natl Acad Sci 112:9573–9578
Lopes TDBM et al (2018) Pre-and postnatal exposure of mice to concentrated urban PM 2.5 decreases the number of alveoli and leads to altered lung function at an early stage of life. Environ Pollut 241:511–520
Ma B, Bates JH (2012) Continuum vs. spring network models of airway-parenchymal interdependence. J Appl Physiol 113:124–129
Maksym GN, Fredberg JJ, Bates JH (1998) Force heterogeneity in a two-dimensional network model of lung tissue elasticity. J Appl Physiol 85:1223–1229
Mead J, Takishima T, Leith D (1970) Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 28:596–608
Mijailovich SM, Stamenovic D, Brown R, Leith DE, Fredberg JJ (1994) Dynamic moduli of rabbit lung tissue and pigeon ligamentum propatagiale undergoing uniaxial cyclic loading. J Appl Physiol 76:773–782
Muiznieks LD, Keeley FW (2013) Molecular assembly and mechanical properties of the extracellular matrix: a fibrous protein perspective. Biochimica et Biophysica Acta (BBA) Mol Basis Dis 1832:866–875
Ochs M et al (2004) The number of alveoli in the human lung. Am J Respir Crit Care Med 169:120–124
Oliveira CL, Bates JH, Suki B (2014) A network model of correlated growth of tissue stiffening in pulmonary fibrosis. New J Phys 16:065022
Rausch S, Martin C, Bornemann P, Uhlig S, Wall W (2011) Material model of lung parenchyma based on living precision-cut lung slice testing. J Mech Behav Biomed Mater 4:583–592
Ritter MC et al (2009) A zipper network model of the failure mechanics of extracellular matrices. Proc Natl Acad Sci 106:1081–1086. https://doi.org/10.1073/pnas.0808414106
Romero FJ, Pastor A, Lopez J, Romero PV (1998) A recruitment-based rheological model for mechanical behavior of soft tissues. Biorheology 35:17–35. https://doi.org/10.1016/S0006-355X(98)00015-8
Sata M, Takahashi K, Sato S, Tomoike H (1995) Structural and functional characteristics of peripheral pulmonary parenchyma in golden hamsters. J Appl Physiol 78:239–246
Sopakayang R, De Vita R (2011) A mathematical model for creep, relaxation and strain stiffening in parallel-fibered collagenous tissues. Med Eng Phys 33:1056–1063
Sugihara T, Martin C, Hildebrandt J (1971) Length-tension properties of alveolar wall in man. J Appl Physiol 30:874–878
Sugihara T, Hildebrandt J, Martin C (1972) Viscoelastic properties of alveolar wall. J Appl Physiol 33:93–98
Suki B (2014) Assessing the functional mechanical properties of bioengineered organs with emphasis on the lung. J Cell Physiol 229:1134–1140
Suki B, Stamenovic D, Hubmayr R (2011) Lung parenchymal mechanics. Compr Physiol 1:1317–1351. https://doi.org/10.1002/cphy.c100033
Takahashi A, Majumdar A, Parameswaran H, Bartolák-Suki E, Suki B (2014) Proteoglycans maintain lung stability in an elastase-treated mouse model of emphysema. Am J Respir Cell Mol Biol 51:26–33
Valipour A et al (2015) Patterns of emphysema heterogeneity Respiration 90:402–411
Vawter D, Fung Y, West J (1978) Elasticity of excised dog lung parenchyma. J Appl Physiol 45:261–269
West JB, Matthews FL (1972) Stresses, strains, and surface pressures in the lung caused by its weight. J Appl Physiol 32:332–345
Wilson TA (1972) A continuum analysis of a two-dimensional mechanical model of the lung parenchyma. J Appl Physiol 33:472–478
Wilson TA (1981) Mechanics of the pressure volume curve of the lung. Ann Biomed Eng 9:439–449
Yi E, Sato S, Takahashi A, Parameswaran H, Blute TA, Bartolák-Suki E, Suki B (2016) Mechanical forces accelerate collagen digestion by bacterial collagenase in lung tissue strips. Front Physiol 7:287
Yuan H, Kononov S, Cavalcante FS, Lutchen KR, Ingenito EP, Suki B (2000) Effects of collagenase and elastase on the mechanical properties of lung tissue strips. J Appl Physiol 89:3–14
Zhou Y et al (2018) Extracellular matrix in lung development, homeostasis and disease. Matrix Biol 73:77–104
Acknowledgements
This work was funded by Internal Faculty of Engineering Scholarships.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iravani, A., Thambyah, A. & Burrowes, K.S. A viscoelastic two-dimensional network model of the lung extracellular matrix. Biomech Model Mechanobiol 19, 2241–2253 (2020). https://doi.org/10.1007/s10237-020-01336-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-020-01336-1