Abstract
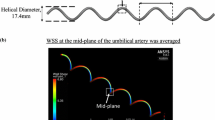
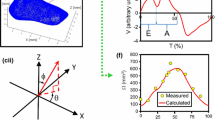
The endothelial cells of the umbilical vessels are frequently used in mechanobiology experiments. They are known to respond to wall shear stress (WSS) of blood flow, which influences vascular growth and remodeling. The in vivo environment of umbilical vascular WSS, however, is not well characterized. In this study, we performed detailed characterization of the umbilical vascular WSS environments using clinical ultrasound scans combined with computational simulations. Doppler ultrasound scans of 28 normal human fetuses from 32nd to 33rd gestational weeks were investigated. Vascular cross-sectional areas were quantified through 3D reconstruction of the vascular geometry from 3D B-mode ultrasound images, and flow velocities were quantified through pulse wave Doppler. WSS in umbilical vein was computed with Poiseuille’s equation, whereas WSS in umbilical artery was obtained via computational fluid dynamics simulations of the helical arterial geometry. Results showed that blood flow velocity for umbilical artery and vein did not correlate with vascular sizes, suggesting that velocity had a very weak trend with or remained constant over vascular sizes. Average WSS for umbilical arteries and vein was 2.81 and 0.52 Pa, respectively. Umbilical vein WSS showed a significant negative correlation with the vessel diameter, but umbilical artery did not show any correlation. We hypothesize that this may be due to differential regulation of vascular sizes based on WSS sensing. Due to the helical geometry of umbilical arteries, bending of the umbilical cord did not significantly alter the vascular resistance or WSS, unlike that in the umbilical veins. We hypothesize that the helical shape of umbilical arteries may be an adaptation feature to render a higher constancy of WSS and flow in the arteries despite umbilical cord bending.











Similar content being viewed by others
References
Adams RH et al (1999) Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev 13:295–306
Adamson SL (1999) Arterial pressure, vascular input impedance, and resistance as determinants of pulsatile blood flow in the umbilical artery. Eur J Obstet Gynecol 84:119–125. doi:10.1016/S0301-2115(98)00320-0
Antiga LaDS (2006) VMTK-vascular modeling toolkit. http://www.vmtk.org
Barbieri C, Cecatti JG, Surita FG, Marussi EF, Costa JV (2012) Sonographic measurement of the umbilical cord area and the diameters of its vessels during pregnancy. J Obstet Gynaecol 32:230–236. doi:10.3109/01443615.2011.647129
Benirschke K, Kaufmann P (1995) Pathology of the human placenta. Springer, New York
Boito S, Struijk PC, Ursem NTC, Stijnen T, Wladimiroff JW (2002) Umbilical venous volume flow in the normally developing and growth-restricted human fetus. Ultrasound Obstet Gynecol 19:344–349. doi:10.1046/j.1469-0705.2002.00671.x
Cheng C et al (2005) Shear stress affects the intracellular distribution of eNOS: direct demonstration by a novel in vivo technique. doi:10.1182/blood-2005-06-2326
de Laat M, Franx A, van Alderen E, Nikkels P, Visser G (2005) The umbilical coiling index, a review of the literature. J Matern Fetal Neonatal Med 17:93–100
Dean WR, Hurst JM (1959) Note on the motion of fluid in a curved pipe. Mathematika 6:77–85. doi:10.1112/S0025579300001947
dela Paz NG, Walshe TE, Leach LL, Saint-Geniez M, D’Amore PA (2012) Role of shear-stress-induced VEGF expression in endothelial cell survival. J Cell Sci 125:831–843
Gardosi J, Clausson B, Francis A (2009) The value of customised centiles in assessing perinatal mortality risk associated with parity and maternal size. BJOG 116:1356–1363. doi:10.1111/j.1471-0528.2009.02245.x
Handwerker SM, Halevy S, Altura BM (1990) Effects of vasoactive agents on isolated human umbilical veins in preeclamptic women. Magnes Trace Elements 9:70–78
Hsieh HJ, Li NQ, Frangos JA (1991) Shear stress increases endothelial platelet-derived growth factor mRNA levels. Am J Physiol 260(2 Pt 2):H642–H646
Jessop FA, Lees CC, Pathak S, Hook CE, Sebire NJ (2014) Umbilical cord coiling: clinical outcomes in an unselected population and systematic review. Virchows Arch 464:105–112. doi:10.1007/s00428-013-1513-2
Jinsuo Z, Benzhao Z (1999) Fluid flow in a helical pipe. Acta Mech Sin 15:299–312. doi:10.1007/BF02487928
Kao HC (1987) Torsion effect on fully developed flow in a helical pipe. J Fluid Mech 184:335–356
Kaplan AD, Jaffa AJ, Timor IE, Elad D (2010) Hemodynamic analysis of arterial blood flow in the coiled umbilical cord. Reprod Sci 17:258–268
Kim JS et al (2008) Gene expression profiling demonstrates a novel role for foetal fibrocytes and the umbilical vessels in human fetoplacental development. J Cell Mol Med 12:1317–1330. doi:10.1111/j.1582-4934.2008.00284.x
Korten S et al (2013) Impact of Hey2 and COUP-TFII on genes involved in arteriovenous differentiation in primary human arterial and venous endothelial cells. Basic Res Cardiol 108:362. doi:10.1007/s00395-013-0362-0
Lawn JE, Cousens S, Zupan J, Steering Lancet Neonatal Survival T (2005) 4 Million neonatal deaths: when? where? why? Lancet 365:891–900. doi:10.1016/S0140-6736(05)71048-5
Lees C, Albaiges G, Deane C, Parra M, Nicolaides KH (1999) Assessment of umbilical arterial and venous flow using color Doppler. Ultrasound Obstet Gynecol 14:250–255. doi:10.1046/j.1469-0705.1999.14040250.x
Liu S, Masliyah JH (1993) Axially invariant laminar flow in helical pipes with a finite pitch. J Fluid Mech 251:315–353
Malek AM, Alper SL, Izumo S (1999) Hemodynamic shear stress and its role in atherosclerosis. JAMA 282:2035–2042
Maulik D (2005) Spectral Doppler: basic principles and instrumentation. In: Maulik D (ed) Doppler ultrasound in obstetrics and gynecology. Springer, New York, pp 19–34
McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu C-M, Russell CG, Chittur KK (2001) DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Nat Acad Sci 98:8955–8960
Misra SEmMsmeWDA et al (2006) Assessment of wall shear stress changes in arteries and veins of arteriovenous polytetrafluoroethylene grafts using magnetic resonance imaging. Cardiovasc Interv Radiol 29:624–629. doi:10.1007/s00270-005-0168-z
Misra S, Fu AA, Misra KD, Glockner JF, Mukhopadhyay D (2009) Wall shear stress measurement using phase contrast magnetic resonance imaging with phase contrast magnetic resonance angiography in arteriovenous polytetrafluoroethylene grafts. Angiology 60:441–447. doi:10.1177/0003319709335908
Nagel T, Resnick N, Dewey CF, Gimbrone MA (1999) Vascular endothelial cells respond to spatial gradients in fluid shear stress by enhanced activation of transcription factors. Arterioscler Thromb Vasc Biol 19:1825–1834. doi:10.1161/01.atv.19.8.1825
Nakatsu MN et al (2003) VEGF(121) and VEGF(165) regulate blood vessel diameter through vascular endothelial growth factor receptor 2 in an in vitro angiogenesis model. Lab Invest 83:1873–1885
Pennati G, Bellotti M, Ferrazzi E, Bozzo M, Pardi G, Fumero R (1998) Blood flow through the ductus venosus in human fetus: calculation using Doppler velocimetry and computational findings. Ultrasound Med Biol 24:477–487. doi:10.1016/S0301-5629(98)00011-8
Raio L, Ghezzi F, Di Naro E, Duwe DG, Cromi A, Schneider H (2003) Umbilical cord morphologic characteristics and umbilical artery Doppler parameters in intrauterine growth-restricted fetuses. J Ultrasound Med 22:1341–1347
Rana J, Ebert GA, Kappy KA (1995) Adverse perinatal outcome in patients with an abnormal umbilical coiling index. Obstet Gynecol 85:573–577
Roach M (1976) The umbilical vessels Perinatal medicine: the basic science underlying clinical practice. Williams and Wilkins, Baltimore
Smiesko V, Kozík J, Dolezel S (1985) Role of endothelium in the control of arterial diameter by blood flow. Blood Vessels 22:247
Snyder B, Hammersley JR, Olson DE (1985) The axial skew of flow in curved pipes. J Fluid Mech 161:281–294. doi:10.1017/S0022112085002932
Strong TH Jr, Jarles DL, Vega JS, Feldman DB (1994) The umbilical coiling index. Am J Obstet Gynecol 170:29–32. doi:10.1016/S0002-9378(94)70378-7
Struijk PC, Stewart PA, Fernando KL, Mathews VJ, Loupas T, Steegers EAP, Wladimiroff JW (2005) Wall shear stress and related hemodynamic parameters in the fetal descending aorta derived from color Doppler velocity profiles. Ultrasound Med Biol 31:1441–1450. doi:10.1016/j.ultrasmedbio.2005.07.006
Struijk PC, Mathews VJ, Loupas T, Stewart PA, Clark EB, Steegers EA, Wladimiroff JW (2008) Blood pressure estimation in the human fetal descending aorta. Ultrasound Obstet Gynecol 32:673–681. doi:10.1002/uog.6137
Takamura H, Kasai H, Arita H, Kito M (1990) Phospholipid molecular species in human umbilical artery and vein endothelial cells. J Lipid Res 31:709–717
Thorburn J, Drummond MM, Whtgham KA, Lowe GDO, Forbes CD, Prentice CRM, Whitfield CR (1982) Blood viscosity and haemostatic factors in late pregnancy, pre-eclampsia and fetal growth retardation. BJOG 89:117–122. doi:10.1111/j.1471-0528.1982.tb04676.x
Traub O, Berk BC (1998) Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol 18:677–685. doi:10.1161/01.atv.18.5.677
Urban G et al (2009) 289: Predicting perinatal outcome through changes in umbilical artery wall distension rate and turbulence index in severely growth-restricted fetuses. Am J Obstet Gynecol 201:S117–S117. doi:10.1016/j.ajog.2009.10.304
van Diik CC, Franx A, de Laat MW, Bruinse HW, Visser GH, Nikkels PG (2002) The umbilical coiling index in normal pregnancy. J Matern fetal Neonatal Med 11:280–283. doi:10.1080/jmf.11.4.280.283
Vinkesteijn ASM, Struijk PC, Ursem NTC, Hop WCJ, Wladimiroff JW (2004) Fetal heart rate and umbilical artery flow velocity variability in intrauterine growth restriction: a matched controlled study. Ultrasound Obstet Gynecol 23:461–465. doi:10.1002/uog.1032
Wang HU, Chen Z-F, Anderson DJ (1998) Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93:741–753. doi:10.1016/S0092-8674(00)81436-1
Wang Y et al (2010) Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465:483–486. http://www.nature.com/nature/journal/v465/n7297/suppinfo/nature09002_S1.html
Yap CH, Liu X, Pekkan K (2014) Characterizaton of the vessel geometry, flow mechanics and wall shear stress in the great arteries of wildtype prenatal mouse. PloS One 9(1):e86878
Yoshizumi M, Kurihara H, Sugiyama T, Takaku F, Yanagisawa M, Masaki T, Yazaki Y (1989) Hemodynamic shear stress stimulates endothelin production by cultured endothelial cells. Biochem Biophys Res Commun 161:859–864. doi:10.1016/0006-291X(89)92679-X
Yoshizumi M, Abe J, Tsuchiya K, Berk BC, Tamaki T (2003) Stress and vascular responses: atheroprotective effect of laminar fluid shear stress in endothelial cells: possible role of mitogen-activated protein kinases. J Pharmacol Sci 91:172–176
Zarins CK, Zatina MA, Giddens DP, Ku DN, Glagov S (1987) Shear stress regulation of artery lumen diameter in experimental atherogenesis. J Vasc Surg 5:413–420. doi:10.1016/0741-5214(87)90048-6
Zhang F, Zhang L, Sun L-l, Meng X-l, Zhao Y, Jin X (2013) Effects of fluid shear stress on expression of smac/DIABLO in human umbilical vein endothelial cells. Curr Ther Res Clin Exp 74:36–40. doi:10.1016/j.curtheres.2012.11.002
Acknowledgments
This study was supported by the NMRC-CRBG-NIG Grant by the Ministry of Health (Grant Number: NMRC/BNIG/2020/2014, PI: Yap Choon Hwai). We thank Nyien Chan Ko Ko for his assistance in the data processing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Saw, S.N., Dawn, C., Biswas, A. et al. Characterization of the in vivo wall shear stress environment of human fetus umbilical arteries and veins. Biomech Model Mechanobiol 16, 197–211 (2017). https://doi.org/10.1007/s10237-016-0810-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-016-0810-5