Abstract
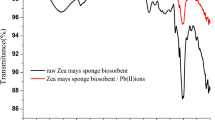
A cyanobacterium isolated from a water sample collected from a coal mine in the West Khasi Hills of Meghalaya, India, and identified as Nostoc sp. (accession no. KX814344) using 16S rRNA analysis showed a high tolerance to chromium. It was shown to be able to grow in the presence of 15 ppm Cr, which is 30 times the highest Cr concentration recorded in the area. Cr biosorption by the cyanobacterium was optimum at pH 6.0 with 3 µg mL−1 biomass. The sorption showed a linear correlation with increasing metal concentration, gradually reaching saturation. An energy dispersive X-ray study verified Cr binding on the cyanobacterial biomass, and FTIR analysis revealed many negatively charged functional groups on the cell surface, which aided in metal binding. Thermodynamic studies showed the biosorption process to be energetically favorable: − 0.479, − 0.665, and − 0.852 kJ mol−1 at temperatures of 293, 303, and 313K, respectively. Sorption isotherm data fit the Langmuir isotherm best, indicating the monolayer nature of the Cr sorption. The organism’s maximum sorption capacity was as high as 20 mg of Cr per g of biomass. The separation factor calculated from the Langmuir isotherm was < 1, signifying favorable interaction between the cyanobacterial biomass and the Cr ions.
抽象
蓝绿藻提取于印度梅加拉亚邦西卡希山(West Khasi Hills)的煤矿水样,经16S rRNA测序鉴定为Nostoc sp.(登记号:KX814344),蓝绿藻表现出很高的耐铬性,可以在铬浓度高于环境30倍的15ppm条件下生存。蓝绿藻的铬生物吸附最佳条件为pH值为6和生物量为3 µg mL-1。吸附能力随金属浓度增大线性提高,逐渐达吸附饱和。能谱分析证实铬确实结合于蓝绿藻群落,傅里叶转换红外光谱(FTIR)分析表明蓝绿藻细胞表面带很多有助于吸附金属的负电荷功能团。热力学研究表明,温度293、303和313K条件下,生物吸附过程有利条件分别为-0.479kJ mol-1、-0.665 kJ mol-1和-0.852 kJ mol-1。吸附数据服从朗缪尔吸附等温线,属于单层铬吸附。每克生物的最大铬生物吸附能力为20 mg (20 mg of Cr per g of biomass)。由朗缪尔吸附等温线计算的分离系数小于1,蓝绿藻与铬离子利于反应。
Zusammenfassung
Aus einer Wasserprobe, entnommen in einer Kohlemine in den West Khasi Hills von Meghalaya (Indien), wurde ein Cyanobakterium isoliert und unter Verwendung der 16S-rRNA-Analyse als Nostoc sp. (GenBank-Zugangs¬nummer KX814344) identifiziert. Dieser Organismus zeigte eine hohe Toleranz gegenüber Chrom. Es wurde gezeigt, dass er in der Lage ist in Gegenwart von 15 ppm Cr zu wachsen, was dem 30-fachen der in diesem Bereich aufgezeichneten Höchstkonzentration an Cr entspricht. Die Cr-Biosorption durch das Cyanobakterium war mit 3 μg mL-1 Biomasse bei pH 6,0 optimal. Die Sorption zeigte eine lineare Korrelation mit steigender Metallkonzentration und erreichte allmählich die Sättigung. Eine energiedispersive Röntgenuntersuchung bestätigte die Cr-Bindung an der cyanobakteriellen Biomasse, und eine FTIR-Analyse zeigte viele negativ geladene funktionelle Gruppen an der Zelloberfläche, die die Metallbindung unterstützten. Thermodynamische Studien veranschaulichten, dass der Biosorptionsprozess energetisch begünstig ist: -0,479 kJ mol-1, -0,665 kJ mol-1 und -0,852 kJ mol-1 bei Temperaturen von 293, 303 bzw. 313 K. Sorptionsisothermen-Daten wurden am besten durch die Langmuir-Isotherme angepasst, was auf den Charakter einer Einzelschicht bei der Cr-Sorption hindeutet. Die maximale Sorptionskapazität des Organismus lag bei 20 mg Cr pro g Biomasse. Der aus der Langmuir-Isotherme berechnete Trennfaktor betrug <1, was eine günstige Wechselwirkung zwischen der cyanobakteriellen Biomasse und den Cr-Ionen anzeigt.
Resumen
Una cianobacteria aislada de una muestra de agua recogida de una mina de carbón en las colinas West Khasi de Meghalaya, India, e identificada como Nostoc sp. (número de acceso KX814344) usando análisis 16S rRNA mostró una alta tolerancia a cromo. Se demostró que podía crecer en presencia de 15 ppm de Cr, que es 30 veces la concentración de Cr más alta registrada en el área. La biosorción de Cr por la cianobacteria fue óptima a pH 6,0 con 3 μg mL-1 de biomasa. La sorción mostró una correlación lineal con el aumento de la concentración de metal, alcanzando gradualmente la saturación. Mediante un estudio de rayos X de energía dispersiva se verificó la unión de Cr en la biomasa de cianobacterias y el análisis FTIR reveló muchos grupos funcionales cargados negativamente en la superficie de la célula que contribuyeron a la unión del metal. Los estudios termodinámicos mostraron que el proceso de biosorción era energéticamente favorable: -0,479 kJ mol-1, -0,665 kJ mol-1 y -0,852 kJ mol-1 a 293, 303 y 313 K, respectivamente. Los datos de isotermas de sorción se ajustan a la isoterma de Langmuir indicando la naturaleza monocapa de la adsorción de Cr. La capacidad de sorción máxima del organismo fue 20 mg Cr/ g de biomasa. El factor de separación calculado a partir de la isoterma de Langmuir fue <1, lo que significa una interacción favorable entre la biomasa cianobacteriana y los iones Cr.








Similar content being viewed by others
References
Ahad RIA, Goswami S, Syiem MB (2017) Biosorption and equilibrium isotherms study of cadmium removal by Nostoc muscorum Meg 1: morphological, physiological and biochemical alterations. 3 Biotech. https://doi.org/10.1007/s13205-017-0730-9
Aksu Z (2001) Equilibrium and kinetic modeling of cadmium (II) biosorption by C. vulgaris in a batch system: effect of temperature. Separ Purif Technol 21:285–294
Anjana K, Kaushik A, Kiran B, Nisha R (2007) Biosorption of Cr(VI) by immobilized biomass of two indigenous strains of cyanobacteria isolated from metal contaminated soil. J Hazard Mater 148:383–386
Arun S, Manikandan NA, Kannan P, Pugazzhenthi G, Syiem MB (2017) Cu(II) removal by Nostoc muscorum and its effect on biomass growth and nitrate uptake: a photobioreator study. Int Biodeter Biodeg 7(01):80–92
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1999) Agarose gel electrophoresis. Short protocols in mol biol, 2nd ed. Wiley, New York City
Bern M, Goldberg D (2005) Automatic selection of representative proteins for bacterial phylogeny. BMC Evol Biol. https://doi.org/10.1186/1471-2148-5-34
Bhanu P, Madhoolika A, Siddharth S (2014) Assessment of air pollution around coal mining area: emphasizing on spatial distributions, seasonal variations and heavy metals, using cluster and principal component analysis. Ind Atmos Pollut Res 5:79–86
Bhattacharjee J (2014) Mining and people’s protest: a study in India’s north east. Int Res J Environ Sci 3(11):65–70
Bulgariu D, Bulgariu L (2013) Sorption of Pb (II) onto a mixture of algae waste biomass and anion exchanger resin in a packed-bed column. Bioresour Technol 129:374e380. https://doi.org/10.1016/j.biotech.2012.10.142
Cepoi L, Zinicovscaia I, Zosim L, Chiriac T, Rudic V, Rudi L, Djur S, Elenciuc D, Miscu V, Ludmil B, Bulimaga V, Gulea A (2016) Metals removal by cyanobacteria and accumulation in biomass. In: Zinicovscaia I, Cepoi L (eds) Cyanobacteria for bioremediation of wastewaters. Springer, Cham, pp 61–111
Chatterjee A, Abraham J (2015) Biosorption capacity of dried spirogyra on heavy metals. Int J Chem Tech Res 8(9):387–392
Deniz F, Saygideger SD, Karaman S (2011) Response to copper and sodium chloride excess in Spirulina sp. (cyanobacteria). Bull Environ Contam Toxicol 87:11–15
Desikachary TV (1959) Cyanophyta. Indian Council of Agricultural Research, New Delhi
Desta MB (2013) Batch sorption experiments: Langmuir and Freundlich isotherm studies for the adsorption of textile metal ions onto agricultural waste. J Thermodyn. https://doi.org/10.1155/2013/375830
Dhal B, Thatoi HN, Das NN, Pandeya BD (2013) Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste. J Hazard Mat 250–251:272–291
Diengdoh OL, Syiem MB, Pakshirajan K, Rai AN (2017) Zn2+ sequestration by Nostoc muscorum: study of thermodynamics, equilibrium isotherms, and biosorption parameters for the metal. Environ Monit Assess 189:314. https://doi.org/10.1007/s10661-017-6013-4
Dixit S, Singh DP (2013) Phycoremediation of lead and cadmium by employing Nostoc muscorum as biosorbent and optimization of its biosorption potential. Int J Phytoremediat 15:801–813
Ertugay N, Bayhan YK (2008) Biosorption of Cr(VI) from aqueous solutions by biomass of Agaricus bisporus. J Hazard Mat 154:432–439
Freundlich H (1906) Adsorption in solutions. J Phys Chem 57:384–410
Gadd GM (2010) Metals, minerals and microbes: geo-microbiology and bioremediation microbiology. Microbiology 156:609–643
Galun M, Galun E,.Siegel BZ, Keller P, Lehr H, Siegel SM (1987) Removal of metal ions from aqueous solutions by Pencillium biomass: kinetic and uptake parameters. Water Air Soil Poll 33:359–371
Gong R, Ding Y, Liu H, Chen Q, Liu Z (2005) Lead biosorption and desorption by intact and pretreated Spirulina maxima biomass. Chemosphere 58:125–130
Goswami S, Diengdoh OL, Syiem MB, Pakshirajan K, Kiran MG (2015a) Zn(II) and Cu(II) removal by Nostoc muscorum: a cyanobacterium isolated from a coal mining pit in Chiehruphi, Meghalaya, India. Can J Microbiol 61:209–215
Goswami S, Syiem MB, Pakshirajan K (2015b) Cadmium removal by Anabaena doliolum Ind1 isolated from a coal mining area in Meghalaya, India: associated structural and physiological alterations. Environ Eng Res 20(1):041–050
Gupta VK, Rastogi A (2008) Sorption and desorption studies of chromium (VI) from nonviable cyanobacterium Nostoc muscorum biomass. J Hazard Mater 154:347–354
Hazarika J, Pakshirajan K, Sinharoy A, Syiem MB (2015) Bioremoval of Cu(II), Zn(II), Pb(II) and Cd(II) by Nostoc muscorum isolated from a coal mining site. J Appl Phycol 27(4):1525–1534
Khambhaty Y, Mody K, Basha S, Jha B (2009) Kinetics, equilibrium and thermodynamic studies on biosorption of hexavalent chromium by dead fungal biomass of marine Aspergillus niger. Chem Eng J 145(3):489–495
Lai MC, Lan EI (2015) Advances in metabolic engineering of cyanobacteria for photosynthetic biochemical production. Metabolites 5:636–658
Langmuir I (1918) Adsorption of gases on plane surfaces of glass, mica and platinum. J Amer Chem Soc 40:1361
Lim TT, Chui PC, Goh KH (2005) Process evaluation for optimization of EDTA use and recovery for heavy metal removal from a contaminated soil. Chemosphere 58:1031–1040
Maheshwari U, Gupta S (2011) Kinetic and equilibrium studies of Cr(VI) removal from aqueous solutions using activated neem bark. Res J Chem Environ 15(2):939–943
Mehta SK, Gaur JP (2005) Use of alga for removing heavy metal ions from wastewater: progress and prospects. Crit Rev Biotechnol 25:113–152
Mohamed ZA (2001) Removal of cadmium and manganese by a non-toxic strain of freshwater cyanobacterium Gloeothece magna. Water Resour 35(18):4405–4409
Nieboer E, Richardson DHS (1980) The replacement of the nondescript term “heavy metals” by a biologically and chemically significant classification of metal ions. Environ Poll B 1:3–26
Nongrum NA, Syiem MB (2012) Effects of copper ion (Cu2+) on the physiological and biochemical activities of the cyanobacterium Nostoc ANTH. Environ Eng Res 17(S1):125–132
Nubel U, Garcia-Pichel F, Muyzer G (1997) PCR Primers to amplify 16S rRNA genes from cyanobacteria. Appl Environ Microbiol 63:3327–3332
Oguz E (2005) Adsorption characteristics and kinetics of the Cr(VI) on the Thuja oriantalis. Colloid Surface A 252:121–128
Ozturk S, Aslim B, Suludere Z, Tan S (2014) Metal removal of cyanobacterial exopolysaccharides by uronic acid content and monosaccharide composition. Carbohydr Polym 101:265–271
Pakshirajan K, Worku AN, Acheampong MA, Lubberding HJ, Lens PNL (2013) Cr(III) and Cr(VI) removal from aqueous solutions by cheaply available fruit waste and algal biomass. Appl Biochem Biotechnol 170:498–513
Reddy DHK, Lee SM (2012) Water pollution and treatment technologies. J Environ Anal Toxicol 2(5):1000e103. https://doi.org/10.4172/2161-0525.1000e103
Rippka R (1988) Recognition and identification of cyanobacteria. In: Packer L, Glazer AN (eds), Cyanobacteria Method Enzymol 167: 28–67
Rippka R, Dereulles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Sahmoune MN, Louhab K, Boukhiarc A (2010) Advanced biosorbents materials for removal of chromium from water and wastewaters. Environ Prog Sustain 30(3):284–293. 10.1002/ep
Singh R, Singh P, Sharma R (2014) Microorganism as a tool of bioremediation technology for cleaning environment. Proc Int Acad Eco Environ Sci 4(1):1–6
Swer S, Singh OP (2003) Coal mining impacting water quality and aquatic biodiversity in Jaintia Hills district of Meghalaya. ENVIS Bull Himal Ecol 11(2):26–33
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Umrania VV (2006) Bioremediation of toxic heavy metals using acido thermophilic autotrophies. Bioresour Technol 97:171–173
WHO (2003) Chromium in drinking-water. Background document for preparation of WHO Guidelines for drinking-water quality. Geneva, World Health Organization (WHO/SDE/WSH/03.04/4)
Acknowledgements
The authors acknowledge the University Grants Commission, New Delhi, Government of India for financial assistance and granting the National Fellowship for Higher Education of ST students. The authors also acknowledge the Department of Chemistry for FTIR facility and SAIF, North Eastern Hill University for GF-AAS and SEM services.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Warjri, S.M., Syiem, M.B. Analysis of Biosorption Parameters, Equilibrium Isotherms and Thermodynamic Studies of Chromium (VI) Uptake by a Nostoc sp. Isolated from a Coal Mining Site in Meghalaya, India. Mine Water Environ 37, 713–723 (2018). https://doi.org/10.1007/s10230-018-0523-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-018-0523-3