Abstract
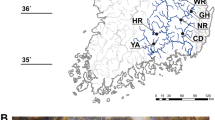
The fluvial eight-barbel loach Lefua sp. 1 is an undescribed species distributed from the Kinki to Chugoku districts, Honshu, and also on Shikoku Island, Japan. Genetic relationships among local populations are unclear and management units remain undetermined. To aid conservation, we determined genetic population structures from microsatellite loci for 20 populations from three river systems on Honshu. The genetic diversity within populations is relatively low; the majority has experienced genetic bottlenecks. Statistical analysis revealed significant divergence among river systems suggesting that each should be recognized as a management unit. Any conservation program should consider the populations’ genetic uniqueness.



Similar content being viewed by others
References
Allendorf FW, Luikart G (2006) Conservation and the genetics of populations. Brackwell, Malden
Aoyama S (2000) A method for the individual identification using the variation in the natural white linear markings on the abdomen of the cobitid fish Lefua sp. Japan J Ichthyol 47:61–65
Aoyama S (2007) Sexual size dimorphism, growth, and maturity of the fluvial eight-barbel loach in the Kako River, Japan. Ichthyol Res 54:268–276
Aoyama S, Doi T (2006) Spawning site of the fluvial eight-barbel loach, Lefua sp., in the natural environment. Ichthyol Res 53:61–65
Aoyama S, Doi T (2011) Morphological comparison of early stages of two Japanese species of eight-barbel loaches: Lefua echigonia and Lefua sp. (Nemacheilidae). Folia Zool 60:355–361
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300
Bohonak AJ (2002) IBD (Isolation By Distance): A program for analyses of isolation by distance. J Hered 93:153–154
Carlsson J, Nilsson J (2000) Population genetic structure of brown trout (Salmo trutta L.) within a northern boreal forest stream. Hereditas 132:173–181
Coleman RA, Weeks AR, Hoffman AA (2013) Balancing genetic uniqueness and genetic variation in determining conservation and translocation strategies: a comprehensive case study of threatened dwarf galaxias, Galaxiella pusilla (Mack) (Pisces: Galaxiidae). Mol Ecol 22:1820–1835
Excoffier L, Lischer H (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10:564–567
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Frankham R (1996) Relationship of genetic variation to population size in wildlife. Conserv Biol 10:1500–1508
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge
Garza JC, Williamson EG (2001) Detection of reduction in population size using data from microsatellite loci. Mol Ecol 10:305–318
Goudet J (1999) PCA-GEN ver 1.2. Population Genetics Laboratory, University of Lausanne, Lausanne, Switzerland
Guo SW, Thompson EA (1992) Performing the exact test of Hardy–Weinberg proportion for multiple alleles. Biometrics 48:361–372
Hosoya K (1993) Cobitidae. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species. Tokai University Press, Tokyo, pp 231–235
Hosoya K (2003) Lefua sp. In: Ministry of the Environment (ed) Threatened wildlife of Japan. Red data book, 2nd edn, vol 4. Pisces: brackish and fresh water fishes. Japan wildlife research center, Tokyo, pp 108–109
Hosoya K (2013) Cobitidae. In: Nakabo T (ed) Fishes of Japan with pictorial keys to the species, 3rd edn. Tokai University Press, Hadano, pp 328–334
Kimura M, Crow JF (1964) The number of alleles that can be maintained in a finite population. Genetics 49:725–738
Kitanishi K, Yamamoto T, Edo K, Higashi S (2012) Influences of habitat fragmentation by damming on the genetic structure of masu salmon populations in Hokkaido, Japan. Conserv Genet 13:1017–1026
Koizumi N, Takahashi H, Minezawa M, Takemura T, Okushima S, Mori A (2007) Isolation and characterization of polymorphic microsatellite DNA markers in the Japanese eight-barbel loach, Lefua echigonia. Mol Ecol Notes 7:836–838
Koizumi N, Watabe K, Gao Z, Mizutani M, Mori A, Takemura T (2008) Preliminary study on genetic population of the Japanese eight-barbel loach in the upper Kokai river basin, Tochigi prefecture using microsatellite DNA. Trans JSIDRE 256:55–61
Luikart G, Allendorf FW, Cornuet J-M, Sherwin WB (1998) Distortion of allele frequency distributions provides a test for recent population bottlenecks. J Hered 89:238–247
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Mihara M, Sakai T, Nakao K, Martins L de O, Hosoya K, Miyazaki J-I (2005) Phylogeography of loaches of the genus Lefua (Balitoridae, Cypriniformes) inferred from mitochondrial DNA sequences. Zool Sci 22:157–168
Miyake T, Nakajima J, Onikura N, Ikemoto S, Iguchi K, Komaru A, Kawamura K (2011) The genetic status of two subspecies of Rhodeus atremius, an endangered bitterling in Japan. Conserv Genet 12:383–400
Miyazaki J-I, Dobashi M, Tamura T, Beppu S, Sakai T, Mihara M, Hosoya K (2011) Parallel evolution in eight-barbel loaches of the genus Lefua (Balitoridae, Cypriniformes) revealed by mitochondrial and nuclear DNA phylogenies. Mol Phyl Evol 60:416–427
Moritz C (1994) Defining ‘Evolutionarily Significant Units’ for conservation. Trends Ecol Evol 9:373–375
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Nei M, Tajima F, Tateno Y (1983) Accuracy of estimated phylogenetic trees from molecular data. J Mol Evol 19:153–170
Piry S, Luikart G, Cornuet J-M (1999) BOTTLENECK: a computer program for detecting recent reductions in the effective population size using allele frequency data. J Hered 90:502–503
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rousset F (1997) Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 145:1219–1228
Ryder OA (1986) Species conservation and systematics: the dilemma of subspecies. Trends Ecol Evol 1:9–10
Saito T, Sakamoto K, Hamaguchi H (2010) Effect verification of fish-way maintenance and adaptable management after construction completed. J JSIDRE 78:11–14
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Saka R, Takehana Y, Suguro N, Sakaizumi M (2003) Genetic population structure of Lefua echigonia inferred from allozymic and mitochondrial cytochrome b variations. Ichthyol Res 50:301–309
Sakai T, Mihara M, Shitara H, Yonekawa H, Hosoya K, Miyazaki J-I (2003) Phylogenetic relationships and intraspecific variations of loaches of the genus Lefua (Balitoridae, Cypriniformes). Zool Sci 20:501–514
Slatkin M (1985) Rare alleles as indicators of gene flow. Evolution 39:53–65
Slatkin M (1995) A measure of population subdivision based on microsatellite allele frequencies. Genetics 139:457–462
Takezaki N, Nei M, Tamura K (2010) POPTREE2: Software for constructing population trees from allele frequency data and computing other population statistics with Windows interface. Mol Biol Evol 27:747–752
van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Waples RS (2015) Testing for Hardy-Weinberg proportions: Have we lost the plot? J Hered 106:1–19
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Yamamoto S, Morita K, Koizumi I, Maekawa K (2004) Genetic differentiation of white-spotted charr (Salvelinus leucomaenis) populations after habitat fragmentation: spatial-temporal changes in gene frequencies. Conserv Genet 5:529–538
Yamashina Y, Kamei T, Hosoya K (1994) Preliminary report on two Lefua species obtained from Hikami district. Hyogo Freshw Biol 45:5–11
Yamazaki Y, Nakamura T, Nishio M, Uehara K (2010) Genetic population structures of the Itasenpara bitterling, Acheilognathus longipinnis, in the Toyama and Osaka regions. Japan J Ichthyol 57:143–148
Yamazaki Y, Yamano A, Oura K (2011) Recent microscale disturbance of gene flow in threatened fluvial lamprey, Lethenteron sp. N, living in a paddy water system. Conserv Genet 12:1373–1377
Yokoyama R, Yamano A, Takeshima H, Nishida M, Yamazaki Y (2009) Disturbance of the indigenous gene pool of the threatened brook lamprey Lethenteron sp. S by intraspecific introgression and habitat fragmentation. Conserv Genet 10:29–43
Acknowledgments
We are grateful to Toshiya Hirowatari of Kyushu University, Kohsuke Akita of Japan Wildlife Research Center, Katsutoshi Watanabe, Yohsuke Kojima, Yo Yamasaki, and Naoyuki Nakahama of Kyoto University, Jun Nakajima of Fukuoka Institute of Health and Environmental Sciences, and Yuichiro Nakayama, Shohei Ueda, Masahiro Suzuki, and Yoshiko Sakamoto of Osaka Prefecture University for their valuable advices. We thank Kazuhiko Uehara and Kazuya Hiramatsu of the Aquatic Life Conservation Research Center, Shuichi Fukuhara of Baika Educational Institution, Nobuyuki Higashiguchi of Suma Aqualife Park, Yuki Taguchi of Asa Zoo, Taiki Ito of Kinki University, Tetsuo Kamei of Zacconekan, and Tatsuya Yokoyama of the Water Museum for providing helpful information about this species. We also thank Ryohei Yamada, Haruka Matsuoka, and the members of the Entomological Laboratory for their help with field surveys.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Inotsuka, A., Hirai, N., Aoyama, S. et al. Genetic population structure of the fluvial eight-barbel loach Lefua sp. 1 in the three river systems in central Honshu, Japan, revealed by microsatellite DNA markers. Ichthyol Res 64, 232–239 (2017). https://doi.org/10.1007/s10228-016-0549-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-016-0549-0