Abstract
Background
Our study aimed to assess whether there was a relationship between clinical benefits and reimbursement decisions as well as the inclusion of economic evaluations in therapeutic positioning reports (IPTs) and to explore factors influencing reimbursement decisions.
Materials and methods
We analysed all anti-cancer drugs approved in Spain from 2010 to September 2022. The clinical benefit of each drug were evaluated using the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) 1.1. The characteristics of these drugs were obtained from the Spanish Agency of Medicines and Medical Devices. Reimbursement status information was obtained using BIFIMED, a web resource available in Spanish and consulted the agreements of the Interministerial Committee on Pricing of Medicines (CIPM).
Results
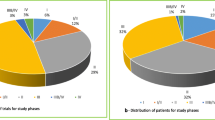
In total, 73 drugs were included involving 197 indications. Almost half of the indications had substantial clinical benefit (49.8% yes vs. 50.3% no). Of the 153 indications with a reimbursement decision, 61 (56.5%) reimbursed indications had substantial clinical benefit compared to 14 (31.1%) of the non-reimbursed (p < 0.01). The median gain of overall survival was 4.9 months (2.8–11.2) for reimbursed indications and 2.9 months (1.7–5) in non-reimbursed (p < 0.05). Only six (3%) indications had an economic evaluation in the IPT.
Conclusion
Our study revealed that there is a relationship between substantial clinical benefit and the reimbursement decision in Spain. However, we also found that the overall survival gain was modest, and a significant proportion of the reimbursed indications had no substantial clinical benefit. Economic evaluations in IPTs are infrequent and cost-effectiveness analysis is not provided by CIPM.




Similar content being viewed by others
References
Fojo, T., Mailankody, S., Lo, A.: Unintended consequences of expensive cancer therapeutics—the pursuit of marginal indications and a me-too mentality that stifles innovation and creativity: the John Conley Lecture. JAMA Otolaryngol Head Neck Surg. 140(12), 1225–1236 (2014). https://doi.org/10.1001/jamaoto.2014.1570
Kumar, H., Fojo, T., Mailankody, S.: An appraisal of clinically meaningful outcomes guidelines for oncology clinical trials. JAMA Oncol. 2(9), 1238–1240 (2016). https://doi.org/10.1001/jamaoncol.2016.0931
Shin, G., Kwon, H.Y., Bae, S.: For whom the price escalates: high price and uncertain value of cancer drugs. Int J Environ Res Public Health. 19(7), 4204 (2022). https://doi.org/10.3390/ijerph19074204
Kemp, R., Prasad, V.: Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med. 15(1), 134 (2017). https://doi.org/10.1186/s12916-017-0902-9
Chen, E.Y., Haslam, A., Prasad, V.: FDA acceptance of surrogate end points for cancer drug approval: 1992–2019. JAMA Intern Med. 180(6), 912–914 (2020). https://doi.org/10.1001/jamainternmed.2020.1097
Haslam, A., Hey, S.P., Gill, J., Prasad, V.: A systematic review of trial-level meta-analyses measuring the strength of association between surrogate end-points and overall survival in oncology. Eur J Cancer. 106, 196–211 (2019). https://doi.org/10.1016/j.ejca.2018.11.012
Fuchs, C.S., Tomasek, J., Yong, C.J., Dumitru, F., Passalacqua, R., Goswami, C. et al; REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro- oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 383(9911), 31–39 (2014). https://doi.org/10.1016/S0140-6736(13)61719-5
Tabernero, J., Yoshino, T., Cohn, A.L., Obermannova, R., Bodoky, G., Garcia-Carbonero, R. et al; RAISE Study Investigators. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first- line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 16(5), 499–508 (2015). https://doi.org/10.1016/S1470-2045(15)70127-0.
Salas-Vega, S., Iliopoulos, O., Mossialos, E.: Assessment of overall survival, quality of life, and safety benefits associated with new cancer medicines. JAMA Oncol. 3(3), 382–390 (2017). https://doi.org/10.1001/jamaoncol.2016.4166
Haslam, A., Herrera-Perez, D., Gill, J., Prasad, V.: Patient Experience Captured by Quality-of-Life Measurement in Oncology Clinical Trials. JAMA Netw Open. 3(3), e200363 (2020). https://doi.org/10.1001/jamanetworkopen.2020.0363.
Hwang, T.J., Gyawali, B.: Association between progression-free survival and patients’ quality of life in cancer clinical trials. Int J Cancer. 144(7), 1746–1751 (2019). https://doi.org/10.1002/ijc.31957
Fiteni, F., Ray, I.L., Ousmen, A., Isambert, N., Anota, A., Bonnetain, F.: Health-related quality of life as an endpoint in oncology phase I trials: a systematic review. BMC Cancer 19(1), 361 (2019). https://doi.org/10.1186/s12885-019-5579-3
Wasalski, E., Mehta, S.: Health-related quality of life data in cancer clinical trials for drug registration: the value beyond reimbursement. JCO Clin Cancer Inform. 5, 112–124 (2021). https://doi.org/10.1200/CCI.20.00100
Godman, B., Bucsics, A., Vella Bonanno, P., Oortwijn, W., Rothe, C.C., Ferrario, A., et al.: Barriers for access to new medicines: searching for the balance between rising costs and limited budgets. Front Public Health. 6, 328 (2018). https://doi.org/10.3389/fpubh.2018.00328
Vokinger, K.N., Hwang, T.J., Grischott, T., Reichert, S., Tibau, A., Rosemann, T., et al.: Prices and clinical benefit of cancer drugs in the USA and Europe: a cost-benefit analysis. Lancet Oncol. 21(5), 664–670 (2020). https://doi.org/10.1016/S1470-2045(20)30139-X
Kelly, R.J., Smith, T.J.: Delivering maximum clinical benefit at an affordable price: engaging stakeholders in cancer care. Lancet Oncol. 15(3), e112–e118 (2014). https://doi.org/10.1016/S1470-2045(13)70578-3
Permanand, G., Bak Pedersen, H.: Managing new premium-priced medicines in Europe. J Pharm Policy Pract. 8(Suppl 1), K2 (2015). https://doi.org/10.1186/2052-3211-8-S1-K2
Agencia Española de Medicamentos y Productos Sanitarios. Medicamentos de Uso Humano. Informes de posicionamiento Terapéutico. Available at: https://www.aemps.gob.es/medicamentos-de-uso-humano/informes-de-posicionamiento-terapeutico/
Cherny, N.I., Sullivan, R., Dafni, U., Kerst, J.M., Sobrero, A., Zielinski, C., et al.: A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti- cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol. 26(8), 1547–1573 (2015). https://doi.org/10.1093/annonc/mdv249
Wild, C., Grössmann, N., Bonanno, P.V., Bucsics, A., Furst, J., Garuoliene, K., et al.: Utilisation of the ESMO-MCBS in practice of HTA. Ann Oncol. 27(11), 2134–2136 (2016). https://doi.org/10.1093/annonc/mdw297
Grössmann, N., Robausch, M., Willenbacher, W., Wolf, S., Simon, J., Wild, C.: „Magnitude of clinical benefit” of solid tumour drugs and their real-world application in the Austrian health care setting. J Cancer Policy. 25, 100235 (2020). https://doi.org/10.1016/j.jcpo.2020.100235
Agencia Española del Medicamento. Informe de Posicionamiento Terapéutico de Tucatinib. 2021. Available at: https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/2021/IPT_49-2021-tucatinib2.pdf
Rojas García, P., Antoñanzas, V.F.: Los contratos de riesgo compartido en el sistema nacional de salud: Percepciones de los profesionales sanitarios [Risk sharing contracts in the national health care system: Perceptions of health care professionals]. Rev Esp Salud Publica 4(92), e201807041 (2018)
Adam, R., Tibau, A., Molto Valiente, C., Šeruga, B., Ocaña, A., Amir, E., et al.: Clinical benefit of cancer drugs approved in Switzerland 2010–2019. PLoS ONE 17(6), e0268545 (2022). https://doi.org/10.1371/journal.pone.0268545
Meyers, D.E., Jenei, K., Chisamore, T.M., Gyawali, B.: Evaluation of the clinical benefit of cancer drugs submitted for reimbursement recommendation decisions in Canada. JAMA Intern Med. 181(4), 499–508 (2021). https://doi.org/10.1001/jamainternmed.2020.8588
Hammerman, A., Greenberg-Dotan, S., Feldhamer, I., Birnbaum, Y., Cherny, N.I.: The ESMO-Magnitude of Clinical Benefit Scale for novel oncology drugs: correspondence with three years of reimbursement decisions in Israel. Expert Rev Pharmacoecon Outcomes Res. 18(1), 119–122 (2018). https://doi.org/10.1080/14737167.2017.1343146
Ambavane, A., Benedict, A., Rivolo, S., Rakonczai, P., Kapetanakis, V.: 1626OESMOMCBS and health technology assessment (HTA): Does value for physicians correspond to value for payers? Ann. Oncol. 30 (Supplement_5) (2019)
Li, J., Vivot, A., Alter, L., Durand-Zaleski, I.: Appraisal of cancer drugs: a comparison of the French health technology assessment with value frameworks of two oncology societies. Expert Rev Pharmacoecon Outcomes Res. 20(4), 405–409 (2020). https://doi.org/10.1080/14737167.2019.1635458
Agencia Española de Medicamentos y Productos Sanitarios. Centro de Información online Medicamentos. Available at: https://cima.aemps.es/cima/publico/home.html
Grössmann, N., Wolf, S., Rothschedl, E., Wild, C.: Twelve years of European cancer drug approval- a systematic investigation of the “magnitude of clinical benefit.” ESMO Open. 6(3), 100166 (2021). https://doi.org/10.1016/j.esmoop.2021.100166
Grössmann, N., Del Paggio, J.C., Wolf, S., Sullivan, R., Booth, C.M., Rosian, K., Emprechtinger, R., Wild, C.: Five years of EMA-approved systemic cancer therapies for solid tumours-a comparison of two thresholds for meaningful clinical benefit. Eur J Cancer. 82, 66–71 (2017). https://doi.org/10.1016/j.ejca.2017.05.029
European Medicines Agency. European Public Assessment Reports. Available at: https://www.ema.europa.eu/en
United States National Library of Medicine. Clinical Trials database. Available at: https://clinicaltrials.gov/
Ministerio de Sanidad. Buscador de la información sobre la situación de financiación de los medicamentos (BIFIMED). Available at: https://www.sanidad.gob.es/profesionales/medicamentos.do
GENESIS-SEFH. Sumario de informes publicados por los hospitales. 2023. Available at: https://gruposdetrabajo.sefh.es/genesis/genesis/Enlaces/InformesHosp_abc.htm?ml=1
Ministerio de Sanidad. Comisión Interministerial de Precios de Medicamentos y Productos Sanitarios. Available at: https://www.sanidad.gob.es/profesionales/farmacia/CIPMyPS.htm
Tibau, A., Molto, C., Ocaña, A., et al.: Magnitude of clinical benefit of cancer drugs approved by the US Food and Drug Administration. J Natl Cancer Inst. 110(5), 486–492 (2018). https://doi.org/10.1093/jnci/djx232
Tibau, A., Molto, C., Borrell, M., et al.: Magnitude of clinical benefit of cancer drugs approved by the US Food and Drug Administration based on single-arm trials. JAMA Oncol. 4(11), 1610–1611 (2018). https://doi.org/10.1001/jamaoncol.2018.4300
Del Paggio, J.C., Azariah, B., Sullivan, R., Hopman, W.M., James, F.V., Roshni, S., Tannock, I.F., Booth, C.M.: Do contemporary randomized controlled trials meet ESMO thresholds for meaningful clinical benefit? Ann Oncol. 28(1), 157–162 (2017). https://doi.org/10.1093/annonc/mdw538
Barnes, T.A., Amir, E., Templeton, A.J., et al.: Efficacy, safety, tolerability and price of newly approved drugs in solid tumors. Cancer Treat Rev. 56, 1–7 (2017)
Davis, C., Naci, H., Gurpinar, E., Poplavska, E., Pinto, A., Aggarwal, A.: Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009–13. BMJ 359, j4530 (2017). https://doi.org/10.1136/bmj.j4530
Adam, R., Tibau, A., Molto Valiente, C., Šeruga, B., Ocaña, A., Amir, E., Templeton, A.J.: Clinical benefit of cancer drugs approved in Switzerland 2010–2019. PLoS ONE 17(6), e0268545 (2022). https://doi.org/10.1371/journal.pone.0268545
Zhang, Y., Naci, H., Wagner, A.K., Xu, Z., Yang, Y., Zhu, J., et al.: Overall Survival Benefits of Cancer Drugs Approved in China From 2005 to 2020. JAMA Netw Open. 5(8), e2225973 (2022). https://doi.org/10.1001/jamanetworkopen.2022.25973
Kovic, B., Jin, X., Kennedy, S.A., Hylands, M., Pedziwiatr, M., Kuriyama, A., et al.: Evaluating Progression-Free Survival as a Surrogate Outcome for Health-Related Quality of Life in Oncology: A Systematic Review and Quantitative Analysis. JAMA Intern Med. 178(12), 1586–1596 (2018). https://doi.org/10.1001/jamainternmed.2018.4710
Gyawali, B., Sharma, S., Booth, C.M.: Is the number of cancer drug approvals a surrogate for regulatory success? J Cancer Policy 22, 100202 (2019). https://doi.org/10.1016/j.jcpo.2019.100202
Pulido S. Solo el 18% de los IPT publicados en 2022 incluyen evaluación económica. El global. 2022 Sept 22. Available at: https://elglobal.es/industria/solo-el-18-de-los-ipt-publicados-en-2022-incluyen-evaluacion-economica/
Hilal, T., Gonzalez-Velez, M., Prasad, V.: Limitations in clinical trials leading to anticancer drug approvals by the US Food and drug administration. JAMA Intern Med. 180(8), 1108–1115 (2020). https://doi.org/10.1001/jamainternmed.2020.2250
Naci, H., Davis, C., Savović, J., Higgins, J.P.T., Sterne, J.A.C., Gyawali, B., et al.: Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014–16: cross sectional analysis. BMJ 366, l5221 (2019). https://doi.org/10.1136/bmj.l5221
Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S et al; CheckMate 648 Trial Investigators. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med. 2022; 386(5): 449–462. https://doi.org/10.1056/NEJMoa2111380.
Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR et al.; Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007; 25(12): 1539–44. doi: https://doi.org/10.1200/JCO.2006.09.6305.
Von Hoff, D.D., Ervin, T., Arena, F.P., Chiorean, E.G., Infante, J., Moore, M., et al.: Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 369(18), 1691–1703 (2013). https://doi.org/10.1056/NEJMoa1304369
West, H., McCleod, M., Hussein, M., Morabito, A., Rittmeyer, A., Conter, H.J., et al.: Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 20(7), 924–937 (2019). https://doi.org/10.1016/S1470-2045(19)30167
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nieto-Gómez, P., Castaño-Amores, C., Rodríguez-Delgado, A. et al. Analysis of oncological drugs authorised in Spain in the last decade: association between clinical benefit and reimbursement. Eur J Health Econ 25, 257–267 (2024). https://doi.org/10.1007/s10198-023-01584-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-023-01584-9