Abstract
The outcomes of preceding fights can influence the probability of winning a subsequent fight, known as the winner/loser effect. However, we know relatively little about how the experience of a preceding fight influences subsequent mating success. Here, we investigated the influence of preceding fight outcomes on subsequent mating behavior in a fruit fly Drosophila prolongata. Subordinate males mated less in two-choice mating assays, showing that the fight outcome predicts male mating success in this species. This tendency remained in a no-choice mating assay where direct interaction between the dominant and subordinate males was eliminated, suggesting that the mating disadvantage of the subordinate males was dependent on the experience of the previous fight rather than the direct interference by the dominant male. When a no-choice mating assay was performed before the fight, the prospective subordinate males mated at the same rate as the dominant males, confirming that the intrinsic male qualities in fighting and mating performances were independent of each other in our experiments. These results indicated that the experience-dependent changes in the subordinate males led to the reduced mating success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The consequences of preceding interactions with other individuals can influence subsequent behavior in various ways. A well-known case is the winner/loser effect in aggression behavior: winners tend to win the next fight, whereas losers become more likely to lose (Hsu et al. 2006). It is also important to know how fight experience influences other behaviors such as subsequent mating (Wong and Candolin 2005). However, many studies that have addressed the relationship between male–male contests and mating success have failed to measure the purely experience-dependent effect of fight outcome in a strict sense for the following two reasons. First, the experimental design in which two males were simultaneously presented to a female (two-choice test) obscures the purely experience-dependent effects by involving direct interaction between the two males. For example, a dominant male may displace a subordinate male from around a female, preventing the subordinate male from having mating opportunities (Harrison et al. 2018). Second, even in no-choice tests in which either a dominant or subordinate male was presented, females may prefer dominant males regardless of the fight outcome because of a cryptic linkage between the probability of winning and female preference. For example, larger males may be more likely to win and are preferred by females because of their body size, not by their experience (Savage et al. 2005). Such an intrinsic linkage between fight outcome and mating success also needs to be discerned from the purely experience-dependent effects on mating success.
The results of the studies that controlled these factors were not in agreement: there was no experience-dependent change in mating success between dominant and subordinate males in house cricket Acheta domesticus, mosquitofish Gambusia holbrooki, earwig Euborellia brunneri, and cricket Gryllus bimaculatus (Savage et al. 2005; Harrison et al. 2018; van Lieshout et al. 2009; Yu et al. 2018), whereas dominant males mated more than subordinate males in fruit fly D. melanogaster, cricket Velarificturus aspersus, and crayfish Faxonius virilis (Zeng et al. 2018; Filice and Dukas 2019; Teseo et al. 2016; Kim et al. 2018; Kola et al. 2021). The different results may be derived from different experimental procedures by which winners and losers were generated (size-matched or unmatched fights). Alternatively, the effects of fight outcome on mating success may be variable between species.
The hierarchy formed by the male–male interaction also influences the use of alternative reproductive tactics—subordinate males may employ distinct mating tactics such as sneaking instead of the normal courtship behavior (Gross 1996). In the theories of the conditional evolutionarily stable strategy, models explaining the switching mechanism of alternative tactics assume that the threshold of the “status” at which tactics switching occurs is genetically determined, and therefore unchangeable within individuals (Gross 1996; Tomkins and Hazel 2007). However, empirical data suggest that reproductive tactics are influenced by prior experience in some cases. For example, ejaculate quality/quantity has been shown in many organisms to be modulated by prior exposure to certain social conditions or social experiences, including male-to-male competition (Felice and Dukas 2019; reviewed by Magris 2021), implying the existence of within-individual plasticity of the threshold for tactics switching. In mosquitofish G. holbrooki, long-term winning experiences made males spend more time with the female and show more mating attempts, indicating that winning experience increased the pre-copulatory investment (Harrison et al. 2022). In male guppies, the experience of social interaction decreased the within-individual variation in the usage of alternative courtship behaviors (courtship display or forced copulation), suggesting that the social experience plastically shifted the threshold for tactics switching, leading to the preferential expression of either tactic in each individual (Polverino et al. 2019). Likewise, subordinate males may switch their mating tactic toward sneaking after the experience of losing a fight. However, no study has examined whether the experience of losing a fight influences the male mating tactics for subsequent mating occasions.
Drosophila prolongata is a fruit fly that has a prominent sexual dimorphism: males are larger, and their forelegs are enlarged (Setoguchi et al. 2014). Males of D. prolongata are highly aggressive and frequently engage in boxing, in which both males rise up on their mid- and hindlegs holding the opponent with forelegs while quickly thrusting their body against the opponent (Kudo et al. 2015, 2017). The forelegs are also used in leg vibration, a species-specific courtship behavior in which males hit the female’s body repeatedly with their forelegs (Setoguchi et al. 2014). Although female mating receptivity is low in D. prolongata compared with other Drosophila species, the copulation rate increases after leg vibration is performed (Setoguchi et al. 2015). In particular, leg vibration is indispensable for non-virgin females to accept further mating (Minekawa et al. 2018, 2020). Therefore, performing leg vibration is advantageous for males to increase their copulation success. At the same time, the sound of leg vibration triggers a type of ‘sneaker’ response of surrounding rival males, which rush to the courted female and intercept it (Setoguchi et al. 2015). Because of this risk, males of D. prolongata generally reduce the rate of performing leg vibration when other males are in close proximity (Setoguchi et al. 2015). Therefore, excluding the other males by aggression from around the female would be beneficial to the courting male. On the other hand, successful interception is often observed when sneaker males do not court females by themselves hiding from a courting male, indicating a possibility that the sneaking behavior is an alternative mating tactic employed by subordinate males, though it has not been examined directly.
Besides leg vibration, female receptivity is also influenced by food availability—females showed a high copulation rate on food, whereas they showed a lower copulation rate without food (Ando et al. 2020). This result suggests the importance of food resources as a mating stage, implying the benefit of territorial behavior over food resources to increase mating opportunities for males. In fact, when two males were put in a chamber, they fought over the food resource and one male stayed on the food surface for a significantly longer time than the other, indicating that a hierarchy was formed between the two males (Amino and Matsuo 2022, 2023). However, it has not been directly shown that the dominant males have more mating opportunities. In another study, a group of males consisting of high- and low-aggression strains were observed with females for their mating behavior, showing that high-aggression males mated more than low-aggression males (Yoshimizu et al. 2022). However, because the actual hierarchy formed among the males were unclarified in this study, it was not directly shown that high-aggression males gained mating success by winning the male–male contest. In fact, the results suggested that the different copulation rates between the male strains were more likely to be due to the difference in courtship behavior (frequency of leg vibration usage) rather than territoriality. Therefore, although the evolution of male-biased body size and male-specific foreleg morphology in D. prolongata strongly suggests that the consequence of male–male contests is tightly linked to the reproductive performance (Kudo et al. 2015; Amino and Matsuo 2020), the immediate mating success of dominant and subordinate males following a contest has not been quantified.
In this study, we conducted two-choice and no-choice mating assays after a male–male competition assay to answer the following questions concerning the effects of fight outcome on mating success in D. prolongata. (1) Do dominant males mate more? (2) Do subordinate males become sneakers? (3) Is the effect of fight outcome on mating success experience-dependent? We used two representative strains that were distinct in their fighting and mating behaviors in our previous screen (Kudo et al. 2015), in case such differences may affect the results.
Methods
Insects and strains
We used two strains, BaVi043 and SaPa014, that show different aggression levels (hereafter referred to as H and L strains, respectively). Males of H strain are highly aggressive and tend to engage in boxing, which persists for several minutes (average ± SE boxing duration = 3.00 ± 0.80 min/30 min of observation; Kudo et al. 2015). In contrast, males of the L strain are less aggressive and rarely engage in boxing (average ± SE boxing duration = 0.28 ± 0.10 min/30 min; Kudo et al. 2015). They use less-intensive forms of aggression such as leg fencing, body thrusts (lunge), and threatening with leg display and wing extension (Kudo et al. 2015, 2017). In courtship, H males perform leg vibration at a higher rate than L males even in the presence of rival males—44% of H males performed leg vibration immediately before copulation, whereas only 10% of L males did so (Matsuo 2018; Setoguchi et al. 2015). There was no difference in the frequency of female interception behavior between the two strains (Yoshimizu et al. 2022). Each strain was the direct descendant of a single wild-captured female. Such a strain harbors limited intrastrain genetic variations, preventing the unintended adaptation to laboratory conditions to occur. Therefore, the difference in fighting and mating strategies, as well as their genetic combinations in a particular strain, is expected to have originated from wild populations (David et al. 2005). In other words, the behavioral differences between H and L strains had not arisen from adaptation or selection in laboratory conditions. To focus on the differences in males, only L strain females were used in all experiments.
Flies were reared on standard cornmeal medium for Drosophila culture (Setoguchi et al. 2014). All cultures were maintained at 20 °C, and all experiments were carried out at the same temperature because the development of D. prolongata is inhibited at higher temperatures (Hitoshi et al. 2016). A 12:12 h light:dark cycle was applied throughout the experiments. In these conditions, the developmental period required from egg to adult was 16 days for females and 18 days for males, and the life span was 2 months for both sexes (Hitoshi et al. 2016; Macartney and Bonduriansky 2021). The density during the developmental stages was controlled by keeping 20 adult males and females in a culture bottle (Drosophila bottles AS355, Thermo Fisher Scientific, Waltham, MA, USA) containing 50 ml of medium for egg laying, and they were transferred to a new bottle every 2 days.
Newly eclosed males were isolated and maintained individually in glass vials (28.5 mm diameter and 95 mm high) with culture medium, therefore they were socially naive until the behavioral assay. Females were maintained in a group of 10 individuals per vial. On the 5th day after eclosion, males were anesthetized on ice and marked blue or yellow on their dorsal surface of thorax using water-based pigment ink (Poska®, Mitsubishi Pencil Co., Ltd., Tokyo, Japan). Marking of each individual was finished within 90 s, and very few individuals (one among 290 preparations) died after recovery.
Observation chamber and video recording
Behavioral observation was carried out on the 7th day after eclosion in a glass chamber (50 mm diameter and 70 mm high), the inner wall of which was treated with silicon polish to prevent the flies from climbing. A disc of wet filter paper was placed at the bottom of the chamber, and a food podium was placed at the center, consisting of the lid of a disposable conical tube (15 ml tube lid, 23 mm diameter and 11 mm high) filled with Drosophila instant medium (Formula 4–24 Drosophila Medium, Carolina Biological Supply Co., Burlington, NC, USA). Up to eight chambers were arranged in two rows, isolated from each other by paper partitions, and covered with glass plates. Fly behavior was recorded using a digital video camera (HDR-CX630V, Sony, Tokyo, Japan) installed 80 cm above the chambers.
Experiment 1: Two-choice assay
This experiment was designed to examine the following possibilities. (1) Do dominant males mate more than subordinate males? (2) Do subordinate males attempt female interception more than dominant males? Two males from the same strain (marked blue or yellow) were introduced into an observation chamber and their behavior was recorded for 1 h (Fig. 1a; contest phase). A female was added into the chamber and their behavior was recorded for an additional 1 h (mating phase). All observations were conducted during the last 2 h of the light phase because the highest locomotor activity was observed either during the first 2 h or last 2 h of the light phase (Yoshimizu et al. 2022). Marking color had no effect on fight outcome (Number of dominant males, blue:yellow = 22:28, binomial test P = 0.480) or copulation rates (copulated/total, blue:11/50, yellow = 16/50, Fisher’s exact test P = 0.368).
Experimental design. a Experiment 1: two-choice assay. Two males were placed in a chamber and observed (recorded) for 1 h. Then a female was added into the chamber and observed for an additional 1 h. b Experiment 2: no-choice assay. Contest-first: two males were placed in a chamber and observed for 1 h. Then, the blue-marked male was transferred to a new chamber by gentle aspiration. A female was added to each chamber and observed for an additional 1 h. Mating-first: a yellow-marked male and a female were placed in a chamber and observed for 1 h. The female was removed by aspiration. Then, a blue marked naive male was added to the chamber and observed for an additional 1 h
Experiment 2: No-choice assay
This experiment was designed to exclude the influence of direct male–male interaction during mating assay to see purely experience-dependent effects on mating behavior. In addition to the contest-to-mating order of assays (Fig. 1b; contest-first assay), mating-to-contest order (mating-first assay) was conducted to examine the following possibilities: (1) If there is an intrinsic link between fight outcome and mating success: e.g., larger males are likely to win and more readily accepted by females. (2) If the experience of successful mating influences the fight outcome. (3) Whether winners gain more mating success (winner effect), or losers decrease mating success (loser effect), compared to that of naive males.
The contest-first assay was performed in the same way as Experiment 1 except for the mating phase where single males were paired with a female. After the 1 h of contest phase, a blue-marked male was aspirated and transferred to a new observation chamber. We fixed the transferred males to be blue-marked ones to avoid the following bias: flies on the floor are much easier to aspirate than those on the food surface. Because the flies on the floor are likely to be losers, it leads to a situation that more losers are selected to be transferred to a new chamber than winners. This was avoided by aspirating only the blue-marked males regardless of their location. Then, a female was introduced into each chamber and mating behavior was recorded for 1 h (mating phase).
The mating-first assay was performed in the reverse order. A yellow marked male was introduced into an observation chamber with a female, and their behavior was recorded for 1 h (mating phase). The females were removed by aspiration, and a blue-marked naive male was added into the chamber. Their behavior was recorded for an additional 1 h (contest phase). No residency effect was observed (dominant:subordinate = 42:36 in yellow males, binomial test P = 0.572).
Video analysis
Video data of the contest phase were analyzed using a custom-made YOLO-based object detection system (Amino and Matsuo 2022, 2023). Briefly, the system detects the position of each individual every second and outputs the results as a table of pixel coordinates in the CSV format. The system also detects the occurrence of boxing and outputs its position in the same CSV file. A dominant male was defined as the individual that spent the longer time on the top surface of the food podium after the two males encountered each other on it. In a separate analysis, we have confirmed that a significant hierarchy was formed between the two males using this criterion—a subordinate male spent significantly shorter time on the food podium than the other (Amino and Matsuo 2022, 2023). If the two males did not encounter the opponent on the food (no encounter), the corresponding data were not used in the subsequent analysis.
Video data of the mating phase were analyzed manually for the occurrence of copulation and female interception. Female interception was defined as a copulation attempt occurred immediately after the other male performed leg vibration.
Statistical analyses
The results were analyzed by fitting a generalized linear mixed model (GLMM) in which the response variable was mating success (copulation rate). The explanatory variables included strain (L or H), fight outcome (dominant or subordinate), and the interaction term between them as fixed effects and the male-pair ID as a random effect. A binomial error distribution and a logit link function were used. For each estimate of coefficients, probability of not being different from zero was examined using the Wäld test. The analysis was performed using R version 4.2.0 with the glmmML library (Broström and Holmberg 2011).
Results
Experiment 1: Two-choice assay
The copulation rate was higher for the dominant males (Fig. 2). In a GLM fitted to the result, the coefficient for fight outcome was negative, supporting the view that the copulation rate was decreased in subordinate males (Table 1 Exp. 1). The frequency of interception attempts was quite low, which occurred only 7 times overall suggesting that less than 10% males adopted the sneaking tactic regardless of the fight outcome (Table 2). The dominant males of the H strain did not attempt interception whereas those of the L strain did, although the difference was not statistically significant between the strains (Fisher’s exact test, P = 0.489), as well as between the dominant and subordinate males (binomial test, P = 0.453), due to the small sample size. Collectively, these results suggest that sneaking is a minor reproductive tactic even among subordinate males.
Experiment 2: No-choice assay
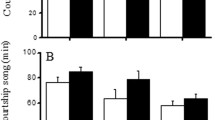
Because the effect of aspiration on copulation rates was negligible (number of copulated males/total males, aspirated: 38/95, intact: 40/95, Fisher’s exact test P = 0.883), the results of both treatments were pooled in the subsequent analyses. In the contest-first assay, the copulation rate of dominant males was higher than that of subordinate males in the H strain but not in the L strain (Fig. 3a). A GLM analysis supported the view that the experience of losing a preceding fight had a significantly negative effect on subsequent mating success (Table 1 Exp. 2 Contest-first).
In the mating-first assay, the copulation rate was not different between prospective dominant and subordinate males (Fig. 3b, Table 1 Exp. 2 Mating-first), showing that fight outcome and mating success is not linked intrinsically (e.g., large body size to win and copulate). The same result also suggested that the experience of successful mating does not increase the probability of winning in the subsequent contest. The copulation rates in the mating-first assay (Fig. 3b) were comparable to that of dominant males in the contest-first assay (Fig. 3a), suggesting that the subordinate males decreased their mating success (loser effect), and the dominant males did not gain more copulations compared with the fight-inexperienced males.
Discussion
Fight outcome and mating success
It has been strongly suggested that the winners of male–male contests in D. prolongata have an advantage over losers in terms of reproductive success, but this has not been confirmed directly. In this study, dominant males mated more than subordinate males in a two-choice assay, confirming that the consequence of male–male contests predicts the subsequent mating success in this species (Fig. 2). An additional no-choice assay revealed that the subordinate males decreased their mating success compared with the naive males, whereas the dominant males maintained the same level of mating success as that of the naive males, suggesting that the loser effect underly the asymmetric copulation rates between the dominant and subordinate males. The no-choice assay in the reverse order (mating first) confirmed that the copulation rate was equal between the prospective dominant and subordinate males, assuring that the observed loser effect was independent of the intrinsic linkage between male qualities in fighting and mating. It would be an interesting next research target to clarify how long the loser effect on mating success lasts in this species. Use of different sizes of fighting chambers would be also helpful to examine the influence of fight intensity on formation of the loser effect.
Intra-species variation of the response to fight outcome
Although the subordinate males showed lower copulation rates in both strains, the difference between the dominant and subordinate males was smaller in the L strain (Fig. 2, 3a). The coefficient for the interaction term between strain and fight outcome was not statistically significant in a GLM fitting analysis (Table 1); therefore, a differential loser effect between the strains was not positively supported by this analysis. Nevertheless, when the dominant and subordinate males were compared in a strain-wise way, P values were substantially large for the L strain (Fisher’s exact test; Experiment 1, L strain: P = 0.143, H strain, P = 0.031; Experiment 2 contest-first, L strain: P = 0.546; H strain: P = 0.049), suggesting that the loser effect was weaker in the L strain. This difference between the two strains leads to an inference that if only one strain had been used for the experiments, the conclusion would have been influenced by the selection of the strain: no effect of fight outcome when the L strain had been selected, or significant loser effect when the H strain had been selected. This situation may occur in other species—we may be led to different conclusions depending on the strain selected for the analysis. Considering that the literature indeed shows bifurcating results among species whether the fight outcome influenced mating success (Savage et al. 2005; Harrison et al. 2018; van Lieshout et al. 2009; Yu et al. 2018; Zeng et al. 2018; Filice and Dukas 2019; Teseo et al. 2016; Kim et al. 2018; Kola et al. 2021), within-species variation may be pervasive in many other species: we may observe different results in each species when we randomly select a strain (or a population with a shared genetic background) for experiments. Because such a heritable component of experience effects should be a subject of natural selection particularly when it influences mating success, it is likely to be involved in the evolutionary mechanisms underlying the experience-dependent modification of subsequent behaviors. For example, different levels of sensitivity to fight outcome may rapidly evolve depending on the surrounding ecological conditions such as density, sex ratio, and resource availability. In fact, artificial selection for shorter duration of loser effect in broad-horned flour beetles (Gnatocerus cornutus) resulted in the loss of loser effect in the second fight within 10 generations (Okada et al. 2019). In this regard, we believe that more attention should be paid in future studies to genetic variation in experience effects on subsequent behaviors.
Fight outcome and alternative reproductive tactics
Examples of the experience-dependent change of courtship behavior are quite rare (Verrell 1983; reviewed by Bretman et al. 2011). In D. prolongata, however, the usage of leg vibration was reduced in the presence of rival males, showing that the courtship behavior of this species can be plastically modulated by social conditions and experiences (Setoguch et al. 2015; Matsuo 2018). In this study, we examined if fight outcome influenced the usage of the sneaking tactic. The result showed that only 10% of males attempted female interception, suggesting that sneaking is a minor tactic even among subordinate males. Nevertheless, it was not excluded yet that subordinate males use sneaking more often than dominant males. Using a two-choice mating assay with size-manipulated males of D. prolongata, Ferreira and Lüpold (2022) showed that smaller males attempted female interception more often than larger males. However, their raw data suggest that the results can be understood in a different way—the choice of alternative tactics was determined by fight outcome, rather than the male body size itself. Although small males attempted interception more often than large males in size-unmatched pairs (attempted interception/individuals, small males: 28/110, large males: 16/110), no difference was observed in size-matched pairs (small males: 19/112, large males: 20/112), suggesting that switching between the alternative tactics was dependent on the consequence of male–male interaction rather than the autonomous body size. In other words, their results support the possibility that subordinate males employ sneaking behavior more often than dominant males in this species.
Conclusions
In this study, we showed that fight outcome influenced the subsequent male mating success in D. prolongata. The subordinate males showed significantly decreased mating success. This effect of fight outcome on mating success was dependent on the experience at least in part, even though the direct male–male interaction that prevents subordinate males from courtship opportunities might also contribute to the results. The experiment in reverse order (mating-first assay) showed that females accepted the prospective dominant and subordinate males equally, proving that the male intrinsic abilities in fighting and mating were independent of each other. Collectively, our experiments demonstrated the loser effect on subsequent mating success in D. prolongata.
Data availability
The raw data are provided as supplementary information.
References
Amino K, Matsuo T (2020) Intra- versus inter-sexual selection on sexually dimorphic traits in Drosophila prolongata. Zoolog Sci 37:210–216. https://doi.org/10.2108/zs200010
Amino K, Matsuo T (2022) Automated behavior analysis using a YOLO-based object detection system. In: Yamamoto D (ed) Behavioral Neurogenetics. Springer, New York, pp 257–275. https://doi.org/10.1007/978-1-0716-2321-3_14
Amino K, Matsuo T (2023) Reproductive advantage of the winners of male-male competition in Drosophila prolongata. Behav Proc 206:104831. https://doi.org/10.1016/j.beproc.2023.104831
Ando Y, Yoshimizu T, Matsuo T (2020) Food availability reverses the effect of hunger state on copulation rate in Drosophila prolongata females. Anim Behav 166:51–59. https://doi.org/10.1016/j.anbehav.2020.06.003
Bretman A, Gage MJG, Chapman T (2011) Quick-change artists: male plastic behavioural responses to rivals. Trends Ecol Evol 26:467–473. https://doi.org/10.1016/j.tree.2011.05.002
Broström G, Holmberg H (2011) Generalized linear models with clustered data: fixed and random effects models. Comput Stat Data Anal 55:3123–3134. https://doi.org/10.1016/j.csda.2011.06.011
David JR, Gibert P, Legout H, Petavy G, Capy P, Moreteau B (2005) Isofemale lines in Drosophila: an empirical approach to quantitative trait analysis in natural populations. Heredity 94:3–12. https://doi.org/10.1038/sj.hdy.6800562
Ferreira JP, Lüpold S (2022) Condition- and context-dependent alternative reproductive tactic in Drosophila prolongata. Behav Ecol 33:213–221. https://doi.org/10.1093/beheco/arab127
Filice DC, Dukas R (2019) Winners have higher pre-copulatory mating success but losers have better post-copulatory outcomes. Proc R Soc B 286:20182838. https://doi.org/10.1098/rspb.2018.2838
Gross MR (1996) Alternative reproductive tactics: diversity within sexes. Trends Ecol Evol 11:92–98. https://doi.org/10.1016/0169-5347(96)81050-0
Harrison LM, Jennions MD, Head ML (2018) Does the winner–loser effect determine male mating success? Biol Let 14:20180195. https://doi.org/10.1098/rsbl.2018.0195
Harrison LM, Vega-Trejo R, Jennions MD (2022) The effect of brief or prolonged bouts of winning or losing male-male contests on plasticity in sexually selected traits. American Naturalist. https://doi.org/10.1086/722829
Hitoshi Y, Ishikawa Y, Matsuo T (2016) Intraspecific variation in heat tolerance of Drosophila prolongata (Diptera: Drosophilidae). Appl Entomol Zool 51:515–520. https://doi.org/10.1007/s13355-016-0425-4
Hsu Y, Earley RL, Wolf LL (2006) Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol Rev 81:33–74. https://doi.org/10.1017/S146479310500686x
Kim Y-K, Saver M, Simon J, Kent CF, Shao L, Eddison M, Agrawal P, Texada M, Truman JW, Heberlein U (2018) Repetitive aggressive encounters generate a long-lasting internal state in Drosophila melanogaster males. PNAS 115:1099–1104. https://doi.org/10.1073/pnas.1716612115
Kola M, Alexander T, Servidio T, Mathews L (2021) Winner and loser effects influence subsequent mating interaction in crayfish. Behev Process 192:104489. https://doi.org/10.1016/j.beproc.2021.104489
Kudo A, Takamori H, Watabe H, Ishikawa Y, Matsuo T (2015) Variation in morphological and behavioral traits among isofemale strains of Drosophila prolongata (Diptera: Drosophilidae). Entomol Sci 18:221–229. https://doi.org/10.1111/ens.12116
Kudo A, Shigenobu S, Kadota K, Nozawa M, Shibata TF, Ishikawa Y, Matsuo T (2017) Comparative analysis of the brain transcriptome in a hyper-aggressive fruit fly, Drosophila prolongata. Insect Biochem Mol Biol 82:11–20. https://doi.org/10.1016/j.ibmb.2017.01.006
Macartney EL, Bonduriansky R (2021) Does female resistance to mating select for live-fast-die-young strategies in males? A comparative analysis in the genus Drosophila. J Evol Biol 35:192–200. https://doi.org/10.1111/jeb.13937
Magris M (2021) Strategic adjustment of ejaculate quality in response to variation of the socio-sexual environment. Behav Ecol Sociobiol 75:91. https://doi.org/10.1007/s00265-021-03032-1
Matsuo T (2018) Effect of social condition on behavioral development during early adult phase in Drosophila prolongata. J Ethol 36:15–22. https://doi.org/10.1007/s10164-017-0524-x
Minekawa K, Miyatake T, Ishikawa Y, Matsuo T (2018) The adaptive role of a species-specific courtship behaviour in coping with remating suppression of mated females. Anim Behav 140:29–37. https://doi.org/10.1016/j.anbehav.2018.04.002
Minekawa K, Amino K, Matsuo T (2020) A courtship behavior that makes monandrous females polyandrous. Evolution 74:2483–2493. https://doi.org/10.1111/evo.14098
Okada K, Okada Y, Dall SR, Hosken DJ (2019) Loser-effect duration evolves independently of fighting ability. Proc R Soc B 286:20190582. https://doi.org/10.1098/rspb.2019.0582
Polverino G, Palmas BM, Evans JP, Gasparini C (2019) Individual plasticity in alternative reproductive tactics declines with social experience in male guppies. Anim Behav 148:113–121. https://doi.org/10.1016/j.anbehav.2018.12.014
Savage KE, Hunt J, Jennions MD, Brooks R (2005) Male attractiveness covaries with fighting ability but not with prior fight outcome in house crickets. Behav Ecol 16:196–200. https://doi.org/10.1093/beheco/arh143
Setoguchi S, Takamori H, Aotsuka T, Sese J, Ishikawa Y, Matsuo T (2014) Sexual dimorphism and courtship behavior in Drosophila prolongata. J Ethol 32:91–102. https://doi.org/10.1007/s10164-014-0399-z
Setoguchi S, Kudo A, Takanashi T, Ishikawa Y, Matsuo T (2015) Social context-dependent modification of courtship behaviour in Drosophila prolongata. Proc R Soc B 282:20151377. https://doi.org/10.1098/rspb.2015.1377
Teseo S, Veerus L, Mery F (2016) Fighting experience affects fruit fly behavior in a mating context. Sci Nat (naturwissenschaften) 103:38. https://doi.org/10.1007/s00114-016-1368-x
Tomkins JL, Hazel W (2007) The status of the conditional evolutionarily stable strategy. Trends Ecol Evol 22:522–528. https://doi.org/10.1016/j.tree.2007.09.002
van Lieshout E, van Wilgenburg E, Elgar MA (2009) No male agonistic experience effect on pre-copulatory mate choice in female earwigs. Behav Ecol Sociobiol 63:1727–1733. https://doi.org/10.1007/s00265-009-0788-4
Verrell P (1983) The influence of the ambient sex-ratio and intermale competition on the sexual behavior of the red-spotted newt, Notophthalmus viridescens (Amphibia, Urodela, Salamandridae). Behav Ecol Sociobiol 13:307–313. https://doi.org/10.1007/BF00299678
Wong BBM, Candolin U (2005) How is female mate choice affected by male competition? Biol Rev 80:559–571. https://doi.org/10.1017/S1464793105006809
Yoshimizu T, Akutsu J, Matsuo T (2022) An indirect cost of male-male aggression arising from female response. Zool Sci 39:514–520. https://doi.org/10.2108/zs210116
Yu V, Shestakov LS (2018) Loser in fight but winner in love: how does inter-male competition determine the pattern and outcome of courtship in cricket Gryllus bimaculatus? Front Ecol Evol 6:197. https://doi.org/10.3389/fevo.2018.00197
Zeng Y, Zhou F-H, Zhu D-H (2018) Fight outcome briefly affects the reproductive fitness of male crickets. Sci Rep 8:9695. https://doi.org/10.1038/s41598-018-27866-4
Funding
Open access funding provided by The University of Tokyo. This research was supported by a Japan Society for the Promotion of Science (JSPS) grant no. 18H02507 to TM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare we have no competing interests.
Ethical approval
Laboratory-maintained insects were used in all experiments. No licences or permits were required for this research, and no ethical approval was required. Nevertheless, we adhered to the ASAB/ABS Guidelines for use and disposal of the animals in this study.
Informed consent
Not applicable to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Toyoshima, N., Matsuo, T. Fight outcome influences male mating success in Drosophila prolongata. J Ethol 41, 119–127 (2023). https://doi.org/10.1007/s10164-023-00778-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-023-00778-1