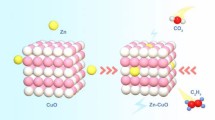
Abstract
Electrochemical reduction of CO2 (CO2RR) is an environmentally benign, cost-effective, and operationally practical approach for utilizing CO2 as a resource. The use of highly efficient electrocatalytic materials is essential for achieving rapid development of CO2RR. In recent years, manganese (Mn)-based oxides have been widely reported as electrocatalytic materials, such as CO2RR catalysts due to their low cost, high electrochemical activity, good stability, and so on. In addition, the valence states and crystal structure types of manganese oxides are both complicated and diversified. To assess the effect of valence state and crystal type on the catalytic performance of CO2RR, this work reviews and summarizes the application of manganese-based oxides as electrocatalytic materials in CO2RR, with structure property as the major line of discussion. On the basis of these findings, a number of ideas and prospects for the design and production of more effective manganese oxide catalysts were also proposed.

(Reproduced with permission from Ref. [29]. Copyright 2020 American Chemical Society)

(Reproduced with permission from Ref. [32]. Copyright 2021 Elsevier)

(Reproduced with permission from Ref. [32]. Copyright 2021 Elsevier)



Similar content being viewed by others
References
Huang HJ, Wang X (2014) Recent progress on carbon-based support materials for elec-trocatalysts of direct methanol fuel cells. J Mater Chem A 2:6266–6291. https://doi.org/10.1007/s00421-008-0955-8
Gautam J, Thanh TD, Maiti K, Kim NH, Lee JH (2018) Highly efficient electrocatalyst of N-doped graphene-encapsulated cobalt-iron carbides towards oxygen reduction reaction. Carbon 137:358–367. https://doi.org/10.1016/j.carbon.2018.05.042
Zheng YB, Zhang Q, Shi J, Li JL, Mei SN, Yu QW, Yang JM, Lyu J (2022) Research progress of catalysts for electrocatalytic reduction of CO2 to various products. Chem Ind Eng Prog 41:1209–1223. https://doi.org/10.16085/j.issn.1000-6613.2021-1936
Wang SH, Tountas AA, Pan WB, Zhao JJ, He L, Sun W, Yang D, Ozin GA (2021) CO2 footprint of thermal versus photothermal CO2 catalysis. Small 17. https://doi.org/10.1002/smll.202007025
Qadir MI, Dupont J (2023) Thermo- and photocatalytic activation of CO2 in ionic liquids nano-domains. Angew Chem Int Edit. https://doi.org/10.1002/anie.202301497
Jiang ZY, Liang XZ, Zheng HL, Liu Y, Wang ZY, Wang P, Zhang XY, Qin XY, Dai Y, Whangbo M, Huang BB (2017) Photocatalytic reduction of CO2 to methanol by three-dimensional hollow structures of Bi2WO6 quantum dots. Appl Catal B Envi-ron 219:209–215. https://doi.org/10.1016/j.apcatb.2017.07.023
Zhang XL, Guo SX, Gandionco KA, Bond AM, Zhang J (2020) Electrocatalytic carbon dioxide reduction: from fundamental principles to catalyst design. Mater Today Adv 7. https://doi.org/10.1016/j.mtadv.2020.100074
Qian X, Deng LF, Wang LF, Shan R, Yuan HR (2019) Recent progress in the electrochemical reduction technology of carbon dioxide. M R 33:102–107
Lu S, Lou FL, Yu ZX (2022) Recent progress in two-dimensional materials for electrocatalytic CO2 reduction. Catalysts 12:228. https://doi.org/10.3390/catal12020228
Li D, Huang Y, Li SM, Wang CH, Li YY, Zhang XT, Liu YC (2020) Thermal coupled photoconductivity as a tool to understand the photothermal catalytic reduction of CO2. Chinese J Catal 41:154–160. https://doi.org/10.1016/S1872-2067(19)63475-3
Zhou J, Liu H, Wang HQ (2022) Photothermal catalysis for CO2 conversion. Chinese Chem Lett 34:107420. https://doi.org/10.1016/j.cclet.2022.04.018
Wang L, Liu XX, Dang YL, Xie HQ, Zhao Q, Ye LQ (2019) Enhanced solar induced photo-thermal synergistic catalytic CO2 conversion by photothermal material decorated TiO2. Solid State Sci 89:67–73. https://doi.org/10.1016/j.solidstatesciences.2018.12.018
Wang ZQ, Yang ZQ, Kadirova ZC, Guo M, Fang RM, He J, Yan YF, Ran JY (2022) Photothermal functional material and structure for photothermal catalytic CO2 reduction: recent advance, application and prospect. Coordin Chem Rev 473:214794. https://doi.org/10.1016/j.ccr.2022.214794
Zhang N, He W, Cheng Z, Lu J, Zhou Y, Ding D, Rong S (2023) Construction of α-MnO2/g-C3N4 Z-scheme heterojunction for photothermal synergistic catalytic decomposition of formaldehyde. Chem Eng J 466. https://doi.org/10.1016/j.cej.2023.143160
Dong YP, Nie R, Wang JX, Yu XG, Tu PC, Chen JZ, Jing HW (2019) Photoelectrocatalytic CO2 reduction based on metalloporphyrin-modified TiO2 photocathode. Chinese J Catal 40:1222–1230. https://doi.org/10.1016/S1872-2067(19)63375-9
Sun ZY, Ma T, Tao HC, Fan Q, Han BX (2017) Fundamentals and challenges of electrochemical CO2 reduction using two-dimensional materials. Chem 3:560–587. https://doi.org/10.1016/j.chempr.2017.09.009
Grills DC, Ertem MZ, McKinnon M, Ngo KT, Rochford J (2018) Mechanistic aspects of CO2 reduction catalysis with manganese-based molecular catalysts. Coord Chem Rev 374:173–217. https://doi.org/10.1016/j.ccr.2018.05.022
Abdi Z, Bagher R, Mohammadi MR, Song ZL, Görlin M, Dau H, Najafpour MM (2021) In situ synthesis of manganese oxide as an oxygen-evolving catalyst: a new strategy. Chem Eur J 27:1330–1336. https://doi.org/10.1002/chem.202002942
Bourre M, Molton F, Chardon-Noblat S, Deronzier A (2011) [Mn(bipyridyl)(CO)3Br]: an abundant metal carbonyl complex as efficient electrocatalyst for CO2 reduction. Angew Chem Int Edit 123:10077–10080. https://doi.org/10.1002/ange.201103616
Scherpf T, Carr CR, Donnelly LJ, Dubrawski ZS, Gelfand BS, Piers WE (2022) A mesoionic carbene-pyridine bidentate ligand improves stability in electrocatalytic CO2 reduction by a molecular manganese catalyst. Inorg Chem 61:13644–13656. https://doi.org/10.1021/acs.inorgchem.2c02689
Petersen HA, Myren THT, Luca OR (2020) Redox-active manganese pincers for electrocatalytic CO2 reduction. Inorganics 8:62. https://doi.org/10.3390/inorganics8110062
Li F, Thevenon A, Rosas-Hernandez A, Wang ZY, Li YL, Gabardo CM, Ozden A, Dinh CT, Li J, Wang YH, Edwards JP, Xu Y, McCallum C, Tao LZ, Liang ZQ, Luo MC, Wang X, Li HH, O’Brien CP, Tan CS, Nam DH, Quintero-Bermudez R, Zhuang TT, Li YC, Han Z, Britt RD, Sinton D, Agapie T, Peters JC, Sargent EH (2020) Molecular tuning of CO2-to-ethylene conversion. Nature 577:509. https://doi.org/10.1038/s41586-019-1782-2
Lee LYC, Wong KY (2017) Electrocatalytic reduction of carbon dioxide. Chem 3:717–718. https://doi.org/10.1016/j.chempr.2017.09.018
Li L, Sun YF, Xie Y (2022) Catalysts design for CO2 electroreduction. Sci China Chem 65:425–427. https://doi.org/10.1007/s11426-021-1137-3
Zhang SY, Shang YY, Zhao RH, Zhao DD, Guo TY, Du JP, Li JP (2022) Research progress on catalysts for electrocatalytic reduction of carbon dioxide to carbon monoxide. Chem Ind and Eng Prog 41:1848–1857. https://doi.org/10.16085/j.issn.1000-6613.2021-0804
Zhu DD, Liu JL, Qiao SZ (2016) Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv Mater 28:3423–3452. https://doi.org/10.1002/adma.201504766
De R, Gonglach S, Paul S, Haas M, Sreejith SS, Gerschel P, Apfel U, Vuong TH, Rabeah J, Roy S, Schöfberger W (2020) Electrocatalytic reduction of CO2 to acetic acid by a molecular manganese corrole complex. Angew Chem Int Edit 59:10527–10534. https://doi.org/10.1002/ange.202000601
Jurss JW, Talukdar K, Roy SS (2020) Electro- and photochemical reduction of CO2 by molecular manganese catalysts: exploring the positional effect of second sphere hydrogen-bond donors. Chem Sus Chem 14:662–670. https://doi.org/10.1002/cssc.202001940
Rønne MH, Cho D, Madsen MR, Jakobsen JB, Eom S, Escoudé É, Hammershøj HCD, Nielsen DU, Pedersen SU, Baik MH, Skrydstrup T, Daasbjerg K (2020) Ligand-controlled product selectivity in electrochemical carbon dioxide reduction using manganese bipyridine catalysts. J Am Chem Soc 142:4265–4275. https://doi.org/10.1021/jacs.9b11806
Wang RJ, Zhao HJ, Qi CR, Zou CR, Liu Y (2022) Research progress on reaction mechanism and catalysts of electrocatalytic reduction of CO2 to single-carbon compounds. Nat Gas Chem Ind 47:11–17
Peng XY, Chen Y, Mi YY, Zhuo LC, Qi GC, Ren JQ, Qiu Y, Liu XJ, Luo J (2019) Efficient electroreduction CO2 to CO over MnO2 nanosheets. Inorg Chem 58:8910–8914. https://doi.org/10.1021/acs.inorgchem.9b01018
Han H, Jin S, Park S, Kim Y, Jang D, Seo MH, Kim WB (2020) Plasma-induced oxygen vacancies in amorphous MnOx boost catalytic performance for electrochemical CO2 reduction. Nano Energy 79:105492. https://doi.org/10.1016/j.nanoen.2020.105492
Zhang YF, Liu JJ, Wei ZX, Tian XX, Ma JM (2019) Single-layer oxygen deficiency δ-MnO2 for electrochemical CO2 reduction. J Electrochem 25. https://doi.org/10.13208/j.electrochem.180945
Zhu N, Zhang X, Chen N, Zhu J, Zheng X, Chen Z, Sheng T, Wu Z, Xiong Y (2023) Integration of MnO2 nanosheets with Pd nanoparticles for efficient CO2 electroreduction to methanol in membrane electrode assembly electrolyzers. J Am Chem Soc 145:24852–24861. https://doi.org/10.1021/jacs.3c09307
Ishida M, Kikkawa S, Hori K, Teramura K, Asakura H, Hosokawa S, Tanaka T (2020) Effect of surface reforming via O3 treatment on the electrochemical CO2 reduction activity of a Ag cathode. Acs Appl Energ Mater 3:6552–6560. https://doi.org/10.1021/acsaem.0c00746
Kwok KS, Wang Y, Cao MC, Shen H, He Z, Poirier G, McCandless BE, Livi KJ, Muller DA, Wang C, Gracias DH (2019) Nano-folded gold catalysts for electroreduction of carbon dioxide. Nano Lett 19:9154–9159. https://doi.org/10.1021/acs.nanolett.9b04564
Chen Z, Cao S, Li J, Yang C, Wei S, Liu S, Wang Z, Lu X (2023) N, S coordination in Ni single-atom catalyst promoting CO2RR towards HCOOH. Phys Chem Chem Phys 25:29951–29959. https://doi.org/10.1039/d3cp03722c
He X-J, Feng J-X, Ren Q, Li G-R (2018) Ni nanoparticle-decorated-MnO2 nanodendrites as highly selective and efficient catalysts for CO2 electroreduction. J Mater Chem A 6:19438–19444. https://doi.org/10.1039/c8ta07687a
Su X, Yang X-F, Huang Y, Liu B, Zhang T (2019) Single-atom catalysis toward efficient CO2 conversion to CO and formate products. Accounts Chem Res 52:656–664. https://doi.org/10.1021/acs.accounts.8b00478
Sun Q, Zhao Y, Tan X, Jia C, Su Z, Meyer Q, Ahmed MI, Zhao C (2023) Atomically dispersed Cu-Au alloy for efficient electrocatalytic reduction of carbon monoxide to acetate. ACS Catal 13:5689–5696. https://doi.org/10.1021/acscatal.2c06145
Zheng M, Zhou X, Wang Y, Chen G, Li M (2023) The facet dependence of CO2 electroreduction selectivity on a Pd3Au bimetallic catalyst: a DFT study. Molecules 28. https://doi.org/10.3390/molecules28073169
Nitopi S, Bertheussen E, Scott SB, Liu X, Engstfeld AK, Horch S, Seger B, Stephens IEL, Chan K, Hahn C, Norskov JK, Jaramillo TF, Chorkendorff I (2019) Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem Rev 119:7610–7672. https://doi.org/10.1021/acs.chemrev.8b00705
Zhang JL, Sun WP, Ding LC, Wu ZC, Gao F (2021) Au Nanocrystals@Defective amorphous MnO2 nanosheets core/shell nanostructure with effective CO2 adsorption and activation toward CO2 electroreduction to CO. ACS Sustain Chem Eng 9:5230–5239. https://doi.org/10.1021/acssuschemeng.1c00995
Li CW, Kanan MW (2012) CO2 Reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J Am Chem Soc 134:7231–7234. https://doi.org/10.1021/ja3010978
Angamuthu R, Byers P, Lutz M, Spek AL, Bouwman E (2010) Electrocatalytic CO2 conversion to oxalate by a copper complex. Science 327:313–315. https://doi.org/10.1126/science.1177981
Roche I, Scott K (2009) Carbon-supported manganese oxide nanoparticles as electrocatalysts for oxygen reduction reaction (orr) in neutral solution. J Appl Electrochem 39:197–204. https://doi.org/10.1007/s10800-008-9653-9
Bai F, Xu L, Wang D, An L, Hao Z, Li F (2021) Effect of the valence state of Mn in MnOx/Ti4O7 composites on the catalytic performance for oxygen reduction reaction and oxygen evolution reaction. RSC Adv 11:1524–1530. https://doi.org/10.1039/d0ra08575h
Siow JH, Bilad MR, Caesarendra W, Leam JJ, Bustam MA, Sambudi NS, Wibisono Y, Mahlia TMI (2021) Progress in development of nanostructured manganese oxide as catalyst for oxygen reduction and evolution reaction. Energies 14. https://doi.org/10.3390/en14196385
Sona J, Songb D, Leec KR, Han J (2019) Electrochemical reduction of CO2 on Ag/MnO2 binary catalyst. J Environ Chem Eng 7:103212. https://doi.org/10.1016/j.jece.2019.103212
Zhang N, Zhang X, Tao L, Jiang P, Ye C, Lin R, Huang Z, Li A, Pang D, Yan H, Wang Y, Xu P, An S, Zhang Q, Liu L, Du S, Han X, Wang D, Li Y (2021) Silver single-atom catalyst for efficient electrochemical CO2 reduction synthesized from thermal transformation and surface reconstruction. Angew Chem Int Ed 60:6170–6176. https://doi.org/10.1002/anie.202014718
Huang ZD, Shang L, Qi HY, Zhao Z, Tu BF, Yang WS, Cheng MJ (2019) Charge transfer reactions in CO2 electroreduction on manganese doped ceria. Chem Electro Chem 6:1668–1672. https://doi.org/10.1002/celc.201801427
Wu M, Ruan Q, Jiang H, Zhang L, Wang D, Zou J (2022) A co-carbonization strategy for confining ultralow-loaded Fe/Mn dual sites in hierarchically porous N-doped carbon for synergistic CO2 electroreduction. J Mater Chem A 10:25463–25470. https://doi.org/10.1039/d2ta06567c
Pandey J, Hua B, Ng W, Yang Y, van der Veen K, Chen J, Geels NJ, Luo JL, Rothenberg G, Yan N (2017) Developing hierarchically porous MnOx/NC hybrid nanorods for oxygen reduction and evolution catalysis. Green Chem 19:2793–2797. https://doi.org/10.1039/c7gc00147a
Wang M, Zhang B, Ding J, Xu N, Bernards MT, He Y, Shi Y (2020) Three-dimensional nitrogen-doped graphene aerogel-supported MnO nanoparticles as efficient electrocatalysts for CO2 reduction to CO. ACS Sustain Chem Eng 8:4983–4994. https://doi.org/10.1021/acssuschemeng.0c01194
Han P, Yu X, Yuan D, Kuang M, Wang Y, Al-Enizi AM, Zheng G (2019) Defective graphene for electrocatalytic CO2 reduction. JCIS 534:332–337. https://doi.org/10.1016/j.jcis.2018.09.036
Kim H-E, Lee IH, Cho J, Shin S, Ham HC, Kim JY, Lee H (2019) Palladium single-atom catalysts supported on C@C3N4 for electrochemical reactions. ChemElectroChem 6:4757–4764. https://doi.org/10.1002/celc.201900772
Meng Y, Gao Y, Li K, Tang H, Wang Y, Wu Z (2021) Transition metal doped C3N monolayer as efficient electrocatalyst for carbon dioxide electroreduction: a computational study. Appl Surf Sci 542:148568. https://doi.org/10.1016/j.apsusc.2020.148568
Mulik BB, Munde AV, Bankar BD, Biradar AV, Sathe BR (2021) Highly efficient manganese oxide decorated graphitic carbon nitrite electrocatalyst for reduction of CO2 to formate. Catal Today 370:104–113. https://doi.org/10.1016/j.cattod.2020.12.008
Acknowledgements
This study was supported by the National NaturalScience Foundation of China (No.21878055).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhai, J., Wu, X., Cai, J. et al. Progress in the electrochemical reduction of CO2 catalyzed by manganese-based oxides. J Mater Cycles Waste Manag (2024). https://doi.org/10.1007/s10163-024-01959-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10163-024-01959-x