Abstract
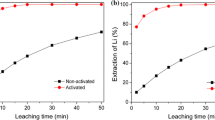
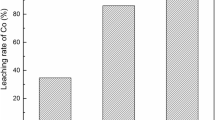
To increase the degree of cobalt (Co) extraction, the process of the cathode material leaching was performed in a sulfuric acid (H2SO4) solution containing sulfur dioxide (SO2) as a reducing agent. To provide a high resolution of the obtained results, frequent monitoring of Co concentrations in leached solution was conducted using an ultraviolet–visible spectrophotometer with several specific modifications related to the connection of the reaction vessel with the instrumental cuvette. The maximum degree of Co leaching (99.4%) was achieved with H2SO4 concentration of 3 mol/L, solid phase concentration of 33 g/L, temperature of 85 °C, SO2 volume flow of 2 L/min, and leaching time of 60 min. The results of the performed kinetic analyses indicated that the Avrami equation best describes the investigated leaching process, which later was supported by the results of X-ray diffraction and scanning electron microscopy–energy-dispersive X-ray spectroscopy analyses. Also, the activation energy of 28 ± 3 kJ/mol is in favor of the fact that the process of Co leaching was controlled by the factors, such as diffusion and chemical reaction. The results of this study indicated that SO2 can be used as an effective reducing agent in the investigated process.










Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article.
References
Sattar R, Ilyas S, Bhatti HN, Ghaffar A (2019) Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Sep Purif Technol 209:725–733. https://doi.org/10.1016/j.seppur.2018.09.019
Tang H, Tan F, Dai X, Qiao Y, Zheng X (2020) Comprehensive recovery of mixed spent of LiNixCoyMn(1–x−y)O2 and LiFePO4. J Mater Cycles Waste Manag 22:1734–1743. https://doi.org/10.1007/s10163-020-01059-6
Zheng X, Zhu Z, Lin X, Zhang Y, He Y, Cao H, Sun Z (2018) A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 4:361–370. https://doi.org/10.1016/j.eng.2018.05.018
Takahashi VCI, Botelho Junior AB, Espinosa DCR, Tenório JAS (2020) Enhancing cobalt recovery from Li-ion batteries using grinding treatment prior to the leaching and solvent extraction process. J Environ Chem Eng 8:103801. https://doi.org/10.1016/j.jece.2020.103801
Chen H, Gu S, Guo Y, Dai X, Zeng L, Wang K, He C, Dodbiba G, Wei Y, Fujita T (2021) Leaching of cathode materials from spent lithium-ion batteries by using a mixture of ascorbic acid and HNO3. Hydrometallurgy 205:105746. https://doi.org/10.1016/j.hydromet.2021.105746
Rarotra S, Kumar P, Satyabrata S, Kumar P, Kim KH (2022) Transformation of recovered cobalt from lithium-ion batteries into zeolitic imidazolate framework-67. J Mater Cycles Waste Manag 24:425–432. https://doi.org/10.1007/s10163-021-01328-y
Badawy SM, Nayl AA, El Khashab RA, El Khateeb MA (2014) Cobalt separation from waste mobile phone batteries using selective precipitation and chelating resin. J Mater Cycles Waste Manag 16:739–746. https://doi.org/10.1007/s10163-013-0213-y
Takacova Z, Havlik T, Kukurugya F, Orac D (2016) Cobalt and lithium recovery from active mass of spent Li-ion batteries: Theoretical and experimental approach. Hydrometallurgy 163:9–17. https://doi.org/10.1016/j.hydromet.2016.03.007
Medić D, Milić S, Alagić S, Đorđević I, Dimitrijević S (2020) Classification of spent Li-ion batteries based on ICP-OES/X-ray characterization of the cathode materials. Hem Ind 74:221–230. https://doi.org/10.2298/HEMIND200114012M
Ndalamo J, Mulaba-Bafubiandi AF, Mamba BB (2011) UV/visible spectroscopic analysis of CO3+ and CO2+ during the dissolution of cobalt from mixed Co-Cu oxidized ores. Int J Min Met Mater 18:260–269. https://doi.org/10.1007/s12613-011-0432-y
Swain B, Jeong J, Jae-chun Lee J-C, Lee G-H, Sohn J-S (2007) Hydrometallurgical process for recovery of cobalt from waste cathodic active material generated during manufacturing of lithium ion batteries. J Power Sources 167:536–544. https://doi.org/10.1016/j.jpowsour.2007.02.046
Kang J, Senanayake G, Sohn J, Shin SM (2010) Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 100:168–171. https://doi.org/10.1016/j.hydromet.2009.10.010
Zhu S, He W, Li G, Zhou X, Zhang X, Huang J (2012) Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation. T Nonferr Metal Soc 22:2274–2281. https://doi.org/10.1016/S1003-6326(11)61460-X
Nadimi H, Karazmoudeh NJ (2020) Leaching of Co, Mn and Ni Using H2O2 in sulfuric acid medium from mobile phone LIBs. J Inst Eng India Ser D 101:111–116. https://doi.org/10.1007/s40033-020-00221-6
Vieceli N, Nogueira CA, Guimarães C, Pereira MFC, Durão FO, Margarido F (2018) Hydrometallurgical recycling of lithium-ion batteries by reductive leaching with sodium metabisulphite. Waste Manage 71:350–361. https://doi.org/10.1016/j.wasman.2017.09.032
Meshram P, Abhilash PBD, Mankhand TR, Devec H (2016) Comparision of different reductants in leaching of spent lithium ion batteries. JOM 68:2613–2623. https://doi.org/10.1007/s11837-016-2032-9
Chen X, Luo C, Zhang J, Kong J, Zhou T (2015) Sustainable recovery of metals from spent lithium-ion batteries: A green process. ACS Sustain Chem Eng 3:3104–3113. https://doi.org/10.1021/acssuschemeng.5b01000
Chen X, Guo C, Ma H, Li J, Zho T, Cao L, Kang D (2018) Organic reductants based leaching: A sustainable process for the recovery of valuable metals from spent lithium ion batteries. Waste Manage 75:459–468. https://doi.org/10.1016/j.wasman.2018.01.021
Wan X, Taskinen P, Shi J, Jokilaakso (2021) A potential industrial waste–waste co-treatment process of utilizing waste SO2 gas and residue heat to recover Co, Ni, and Cu from copper smelting slag. J Hazard Mater 414:125541. doi:https://doi.org/10.1016/j.jhazmat.2021.125541
Guo Y, Zhao YL, Lou X, Zhou T, Wang Z, Fang C, Guan J, Chen S, Xu X, Zhang RQ (2020) Efficient degradation of industrial pollutants with sulfur (IV) mediated by LiCoO2 cathode powders of spent lithium ion batteries: A “treating waste with waste” strategy. J Hazard Mater 123090 doi:https://doi.org/10.1016/j.jhazmat.2020.123090
Ren J, Li R, Liu Y, Cheng Y, Mu D, Zheng R, Liu J, Dai C (2017) The impact of aluminum impurity on the regenerated lithium nickel cobalt manganese oxide cathode materials from spent LIBs. New J Chem 41:10959–10965. https://doi.org/10.1039/C7NJ01206C
Peng C, Lahtinen K, Medina E, Kauranen P, Karppinen M, Kallio T, Wilson BP, Lundström M (2020) Role of impurity copper in Li-ion battery recycling to LiCoO2 cathode materials. J Power Sources 450:227630. https://doi.org/10.1016/j.jpowsour.2019.227630
Fu Y, He Y, Qu L, Feng Y, Li J, Liu J, Zhang G, Xie W (2019) Enhancement in leaching process of lithium and cobalt from spent lithium-ion batteries using benzenesulfonic acid system. Waste Manage 88:191–199. https://doi.org/10.1016/j.wasman.2019.03.044
Nayaka GP, Zhan Y, Dong P, Wang D, Pai KV, Manjanna J, Santhosh G, Duan J, Zhou Z, Xiao J (2018) Effective and environmentally friendly recycling process designed for LiCoO2 cathode powders of spent Li-ion batteries using mixture of mild organic acids. Waste Manage 78:51–57. https://doi.org/10.1016/j.wasman.2018.05.030
Golmohammadzadeh R, Rashchi F, Vahidi E (2017) Recovery of lithium and cobalt from spent lithium-ion batteries using organic acids: process optimization and kinetic aspects. Waste Manage 64:244–254. https://doi.org/10.1016/j.wasman.2017.03.037
Meng Q, Zhang Y, Dong P (2017) Use of glucose as reductant to recover Co from spent lithium ions batteries. Waste Manage 64:214–218. https://doi.org/10.1016/j.wasman.2017.03.017
Chernyaev A, Partinen J, Klemettinen L, Wilson BP, Jokilaakso A, Lundström M (2021) The efficiency of scrap Cu and Al current collector materials as reductants in LIB waste leaching. Hydrometallurgy 203:105608. https://doi.org/10.1016/j.hydromet.2021.105608
Das TN (2005) Saturation Concentration of Dissolved O2 in Highly Acidic Aqueous Solutions of H2SO4. Ind Eng Chem Res 44:1660–1664. https://doi.org/10.1021/ie049539m
Yang J, Jiang LX, Liu FY, Jia M, Lai YQ (2020) Reductive acid leaching of valuable metals from spent lithium-ion batteries using hydrazine sulfate as reductant. Trans Nonferrous Met Soc China 30:2256–2264. https://doi.org/10.1016/S1003-6326(20)65376-6
Gao W, Zhang X, Zheng X, Lin X, Cao H, Zhang Y, Sun ZHI (2017) Lithium carbonate recovery from cathode scrap of spent lithium-ion battery: A closed-loop process. Environ Sci Technol 51:1662–1669. https://doi.org/10.1021/acs.est.6b03320
Jha MK, Kumari A, Jha AK, Kumar V, Hait J, Pandey BD (2013) Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone. Waste Manag 33:1890–1897. https://doi.org/10.1016/j.wasman.2013.05.008jha
Jiang F, Chen Y, Ju S, Zhu Q, Zhang L, Peng J, Wang X, Miller JD (2018) Ultrasound-assisted leaching of cobalt and lithium from spent lithium-ion batteries. Ultrason Sonochem 48:88–95. https://doi.org/10.1016/j.ultsonch.2018.05.019
Lin M, Pei ZY, Lei SM, Liu YY, Xia ZJ, Xie FX (2018) Trace muscovite dissolution separation from vein quartz by elevated temperature and pressure acid leaching using sulphuric acid and ammonia chloride solutions. Physicochem Probl Mi 54:448–458. https://doi.org/10.5277/ppmp1839
Zhang C, Wang S, Cao ZF, Zhong H (2018) Kinetics and mechanism of one-step reductive leaching of manganese oxide ores by EDTA/EDTA-2Na. Physicochem Probl Miner Process 54:858–867. https://doi.org/10.5277/ppmp1887
Muzayanha SU, Yudha CS, Hasanah LM, Gupita LT, Widiyandari H, Purwanto A (2020) Comparative study of various kinetic models on leaching of NCA cathode material. Indones J Chem 20:1291–1300. https://doi.org/10.22146/ijc.49412
Li L, Zhai L, Zhang X, Lu J, Chen R, Wu F, Amine K (2014) Recovery of valuable metals from spent lithium-ion batteries by ultrasonic-assisted leaching process. J Power Sources 262:380–385. https://doi.org/10.1016/j.jpowsour.2014.04.013
Partinen J, Halli P, Helin S, Wilson BP, Lundström M (2022) Utilizing Cu+ as catalyst in reductive leaching of lithium-ion battery cathode materials in H2SO4–NaCl solutions. Hydrometallurgy 208:105808. https://doi.org/10.1016/j.hydromet.2021.105808
Cerrillo-Gonzalez MM, Villen-Guzman M, Acedo-Bueno LF, Rodriguez-Maroto JM, Paz-Garcia JM (2020) Hydrometallurgical extraction of Li and Co from LiCoO2 particles-experimental and modeling. Appl Sci 10:6375. https://doi.org/10.3390/app10186375
Dehaine Q, Tijsseling LT, Glass HJ, Törmӓnen T, Butcher AR (2021) Geometallurgy of cobalt ores: A review. Miner Eng 160:106656. https://doi.org/10.1016/j.mineng.2020.106656
Serbula SM, Milosavljevic JS, Kalinovic JV, Kalinovic TS, Radojevic AA, Apostolovski Trujic TLJ, Tasic VM (2021) Arsenic and SO2 hotspot in South-Eastern Europe: An overview of the air quality after the implementation of the flash smelting technology for copper production. Sci Total Environ 777:145981. https://doi.org/10.1016/j.scitotenv.2021
Waste management program of the Republic of Serbia for the period 2022–2031 https://www.ekologija.gov.rs/dokumenta/upravljanje-otpadom/program Accessed 12 November 2022
Acknowledgements
The research presented in this paper was done with the financial support of the Ministry of Education, Science and Technological Development of the Republic of Serbia, within the funding of the scientific research work at the University of Belgrade, Technical Faculty in Bor, according to the contract number 451-03-68/2022-14/200131, and within the funding of the scientific research work at the Mining and Metallurgy Institute Bor, according to the contract number 451-03-68/2022-14/200052.
Funding
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja, grant number 451-03-68/2022-14/200131, Dragana Medic, Maja Nujkić, Snežana Milić and Slađana Alagić, grant number 451-03-68/2022-14/200052 Stefan Đorđievski.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Medić, D.V., Sokić, M.D., Nujkić, M.M. et al. Cobalt extraction from spent lithium-ion battery cathode material using a sulfuric acid solution containing SO2. J Mater Cycles Waste Manag 25, 1008–1018 (2023). https://doi.org/10.1007/s10163-022-01580-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-022-01580-w