Abstract
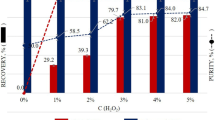
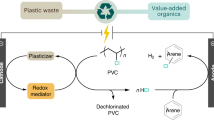
Liquid-phase treatment of waste Poly Vinyl Chloride (PVC) was performed. Chlorine change rate was high when using alcohol, cresol and terpene compared with that observed in the treatment without solvent, so the importance of solvent for dechlorination from PVC was confirmed. By the treatment in alcohol, generation of porous structure was confirmed, which were not observed in the treatment of PVC alone. We were able to propose a method of utilizing carbon resources after chlorine is removed from a different perspective than previous reports. The number of pores and pore size were controlled by adding CO2 gas and basic compound, especially Na2CO3 in 1-Butanol. It indicated that these elements were effective for the production of porous structure based on the liquid-phase treatment. On the other hand, solubilization rate reached near 90% by using terpene at 300 °C in air atmosphere. The most effective solvent for solubilization was o-Cresol and solubilization rate reached to 99.8% at 250 °C in air atmosphere. This result indicates that PVC can be completely decomposed by these solvents in the presence of oxygen. From this research, it is possible to utilize and decompose solid residue by selecting the conditions.












Similar content being viewed by others
References
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv 3:e1700782
Supplementary Materials for Production, use, and fate of all plastics ever made
Basic knowledge of plastic recycling (in Japanese) (https://www.pwmi.or.jp/pdf/panf1.pdf)
Vinyl Environmental Council home page (in Japanese) (http://www.vec.gr.jp/enbi/enbi2_3.html)
Ma S, Jun Lu, Gao J (2002) Study of the low temperature pyrolysis of PVC. Energy Fuels 16:338–342
Ben Gui Yu, Qiao DW, Liu S, Han Z, Yao H, Minghou Xu (2013) Nascent tar formation during polyvinylchloride (PVC) pyrolysis. Proc Combust Inst 34:2321–2329
Lingen Z, Zhenming X (2017) C, H, Cl and in element cycle in wastes: vacuum pyrolysis of PVC plastic to recover indium in LCD panels and prepare carbon coating. ACS Sustain Chem Eng 5:8918–8929
Aboulkas A, El Harfi K (2009) Co-pyrolysis of olive residue with poly (vinyl chloride) using thermogravimetric analysis. J Therm Anal Calorim 95(3):1007–1013
Czégény Z, Jakab E, Bozi J, Blazsó M (2015) Pyrolysis of wood – PVC mixtures. Formation of chloromethane from lignocellulosic materials in the presence of PVC. J Anal Appl Pyrol 113:123–132
Zhou H, Long Y, Meng A, Li Q, Zhang Y (2015) Interactions of three municipal solid waste components during co-pyrolysis. J Anal Appl pyrolysis 111:265–271
Bittencourt PRS, Scremin FR (2019) Evolved gas analysis of PE:PVC systems thermodegradation under inert and oxidizing atmosphere. J Polym Environ 27:612–617
Lopez A, de Marco I, Caballero BM, Laresgoiti MF, Adrados A (2011) Dechlorination of fuels in pyrolysis of PVC containing plastic wastes. Fuel Process Technol 92:253–260
Miskolczi N, Bartha L, Angyal A (2009) Pyrolysis of polyvinyl chloride (PVC)-containing mixed plastic wastes for recovery of hydrocarbons. Energy Fuels 23:2743–2749
Fedorov AA, Chekryshkin YuS, Rudometova OV, Vnutskikh ZhA (2008) Application of inorganic compounds at the thermal processing of polyvinylchloride. Russ J Appl Chem 81(9):1673–1685
Cheng WH, Liang YC (2000) Catalytic pyrolysis of polyvinylchloride in the presence of metal chloride. J Appl Polym Sci 77(11):2464–2471
Blazo M, Lakab E (1999) Effect of metals, metal oxides, and carboxylates on the thermal decomposition processed of poly (vinyl chloride). J Anal Appl Pyrol 49:125–143
Nisar J, Khan MS, Iqbal M, Shah A, Ali G, Sayed M, Khan RA, Shah F, Mahmood T (2018) Thermal decomposition study of polyvinyl chloride in the presence of commercially available oxides catalysts. Adv Polym Technol 37:2336–2343
Kubátová A, Lagadec AJ, Hawthorne SB (2002) Dechlorination of lindane, dieldrin, tetrachloroethane, trichloroethene, and PVC in subcritical water. Environ Sci Technol 36(6):1337–1343
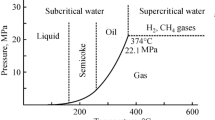
Takeshita Y, Kato K, Takahashi K, Sato Y, Nishi S (2004) Basic study on treatment of waste polyvinyl chloride plastics by hydrothermal decomposition in subcritical and supercritical regions. J Supercrit Fluids 31:185–193
Xiu F-R, Yongwei Lu, Qi Y (2020) DEHP degradation and dechlorination of polyvinyl chloride waste in subcritical water with alkali and ethanol: a comparative study. Chemosphere 249:126138
Xiu F-R, Wang Y, Xuan Yu, Li Y, Yongwei Lu, Zhou Ke, He J, Song Z, Gao X (2020) A novel safety treatment strategy of DEHP-rich flexible polyvinyl chloride waste through low-temperature critical aqueous ammonia treatment. Sci Total Environ 708:134532
Zoukal Z, Elhasri S, Carvalho A, Schmutz M, Collin D, Vakayil PK, Ajayaghosh A, Guenet J-M (2019) Hybrid materials from poly (vinyl chloride) and organogels. ACS Appl Polym Mater 1:1203–1208
Kameda T, Fukuda Y, Grause G, Yoshioka T (2009) Chemical modification of rigid poly (vinyl chloride) by the substitution with nucleophiles. J Appl Polym Sci 116:36–44
Sugeno T, Tagaya H (2015) The effect of solvents on the chemical decomposition of foamed phenol resin in high-temperature conditions. J Master Cycles Waste Manag 17:453–458
Yamashita D, Usui K, Takahashi T, Akutagawa K, Hojo M, Hironaka K, Tagaya H (2020) Chemical recycling of waste tire in high-temperature organic fluid. J Master Cycles Waste Manag 22:1249–1257
Alfonsi K, Colberg J, Dunn PJ, Fevig T, Jennings S, Johnson TA, Peter Kleine H, Knight C, Nagy MA, Perry DA, Stefaniak M (2008) Green chemistry tools to influence chemistry and research chemistry based organisation. Green Chem 10:31–36
Prat D, Wells A, Hayler J, Helen Sneddon C, McElroy R, Abou-Shehada S, Dunn PJ (2016) CHEM21 selection guide of classical- and less classical-solvents. Green Chem 18:288–296
Curzons AD, Constable DC, Cunningham VL (1999) Solvent selection guide: a guide to the integration of environmental, health and safety criteria into the selection of solvents. Clean Prod Processes 1:82–90
Masahiro O (2007) Polymer porous body created by foaming (in Japanese). Polymer 56:70–73
Song W, Barber K, Lee K-Y (2017) Heat-induced bubble expansion as a route to increase the porosity of foam-templated bio-based macroporous polymers. Polymer 118:97–106
Kong W-L, Bao J-B, Wang J, Guo-Hua Hu, Yang Xu, Zhao L (2016) Preparation of open-cell polymer foams by CO2 assisted foaming of polymer blends. Polymer 90:331–341
Forest C, Chaumont P, Cassagnau P, Swoboda B, Sonntag P (2015) Polymer nano-foams for insulating applications prepared from CO2 foaming. Prog Polym Sci 41:122–145
Jin F-L, Zhao M, Park M, Park S-J (2019) Recent trends of foaming in polymer processing: a review. Polymers 11:953
Yamashita J, Shioya M, Kikutani T, Hashimoto T (2001) Activated carbon fibers and films derived from poly (vinylidene fluoride). Carbon 39:207–214
Abe I (1999) Multifunctional materials: activated carbon, charcoal (in Japanese). Shikizai 72(6): 388–396 (https://www.jstage.jst.go.jp/article/shikizai1937/72/6/72_388/_pdf)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10163_2020_1153_MOESM1_ESM.pdf
Fig.S1 (a) FT - IR spectrum, (b) TG curve of the solid residue after the treatment of PVC alone at 230 °C for 2 h (PDF 226 KB)
10163_2020_1153_MOESM3_ESM.pdf
Fig.S3 TG curves of the solid residue after the treatment in alcohol at 200 °C. (a) 1-Propanol, (b) 1-Butanol, (c) 1-Heptanol, (d) 1-Decanol (pdf 719 KB)
10163_2020_1153_MOESM4_ESM.pdf
Fig.S4 SEM images of the solid residue after the treatment in (a) 1-Propanol, (b) 1-Butanol (Small material size), (c) 1-Pentanol, (d) 1-Hexanol, (e) 1-Heptanol, (f) 1-Octanol and (g) shows the digitization for images Fig.9b-I, c, Fig.S5b-I, c–e-I, f-I, *(b) (I): One side, (II): The other side, *(e) (I): One side, (II): The other side, *(f) (I): One side, (II): The other side (pdf 1536 KB)
10163_2020_1153_MOESM5_ESM.pdf
Fig.S5 SEM images of the solid residue after the treatment in various conditions. (a) 1-Butanol + CO2, (b) 1-Decanol + CO2, (c) 1-Butanol + NaHCO3 (0.3eq), (d) 1-Decanol + NaHCO3 (0.3eq), (e) 1-Butanol + Na2CO3(0.3eq), (f) 1-Decanol + Na2CO3 (0.3eq), *(c) (I): One side, (II): The other side, *(d): (I): Part of production of pore, (II): One side, (III) The other side (pdf 1227 KB)
Rights and permissions
About this article
Cite this article
Nozue, K., Tagaya, H. Chemical recycling of waste Poly Vinyl Chloride (PVC) by the liquid-phase treatment. J Mater Cycles Waste Manag 23, 489–504 (2021). https://doi.org/10.1007/s10163-020-01153-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-020-01153-9