Abstract
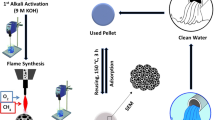
In the presented work, waste glass was converted into highly porous material through a thermo-chemical reaction. In this conversion, lime was used as flux, salt as a medium and soda as a reactant. The effect of relative concentration of fluxes and reactants on the physicochemical properties of the resulting material were investigated at different temperatures. The porous material product was investigated for its textural and chemical nature using scanning electron microscopy (SEM), energy dispersive X-ray analysis (EDX), Fourier Transform infrared spectroscopy (FT-IR) and X ray florescence spectroscopy. SEM analysis showed that the adsorbent had mesoporous and microporous nature. Since porous glass of well-defined pore size and porosity level was fabricated through a hetero-coagulation template processing, the strategy can be developed into a general pathway to prepare various porous inorganic materials with well-defined pore structure. The porous product was also used as an adsorbent for the removal of direct blue dye from aqueous medium. The glass adsorbent significantly removed the Direct blue dye from the aqueous medium. It was observed that the adsorbent can remove 1.27 mg/g of dye after 25 min of treatment.












Similar content being viewed by others
References
Pontikes Y, Christogerou A, Angelopoulos GN, Rambaldi E, Esposito L, Tucci A (2005) Use of scrap soda–lime–silica glass in traditional ceramics. Glass Technol 46:200–206
Pontikes Y, Esposito L, Tucci A, Angelopoulos GN (2007) Thermal behaviour of clays for traditional ceramics with soda–lime–silica waste glass admixture. J. Eur Ceram Soc 27:1657–1663
Shayan A, Xu A (2006) Performance of glass powder as a pozzolanic material in concrete: A field trial on concrete slabs. Cem Concr Res 36:457–468
Lin KL, Huang WJ, Shie JL, Lee TC, Wang KS, Lee CH (2009) The utilization of thin film transistor liquid crystal display waste glass as a pozzolanic material. J Hazard Mater 163:916–921
Shi C, Wu Y, Riefler C, Wang H (2005) Characteristics and pozzolanic reactivity of glass powders. Cem Concr Res 35:987–993
Bernardo E, Esposito L, Rambaldi E, Tucci A, Hreglich S (2008) Recycle of waste glass into glass–ceramic stoneware. J Am Ceram Soc 91:2156–2162
Dondi M, Guarini G, Raimondo M, Zanelli C (2009) Recycling PC and TV waste glass in clay bricks and roof tiles. Waste Manag 29:1945–1951
Loryuenyong V, Panyachai T, Kaewsimork K, Siritai C (2009) Effects of recycled glass substitution on the physical and mechanical properties of clay bricks. Waste Manag 29:2717–2721
Shi C, Zheng K (2007) A review on the use of waste glasses in the production of cement and concrete. Resour Conserv Recycl 52:234–247
Shao Y, Lefort T, Moras S, Rodriguez D (2000) Studies on concrete containing ground waste glass. Cem Concr Res 30:91–100
Kizinievič O, Kizinievič V, Boris R, Girskas G, Malaiškienė J (2018) Eco-efficient recycling of drinking water treatment sludge and glass waste: development of ceramic bricks. J Mater Cycles Waste Manag 20:1228–1238
Ledakowicz S, Solecka M, Zylla RJ (2001) Biodegradation, decolourisation and detoxification of textile wastewater enhanced by advanced oxidation processes. J Biotechnol 89:175–184
El-Aal SEA, Hegazy EA, AbuTaleb MF, Dessouki AM (2005) Radiation synthesis of copolymers for adsorption of dyes from their industrial wastes. J Appl Polym Sci 96:753–763
Lee JW, Choi SP, Thiruvenkatachari R (2006) Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dye Pigment 69:196–203
Zada N, Khan I, Saeed K (2017) Synthesis of multiwalled carbon nanotubes supported manganese and cobalt zinc oxides nanoparticles for the photodegradation of Malachite green. Sep Sci Technol 52:1477–1485
Ratnasamy P, Srinivas D, Knözinger H (2004) Active sites and reactive intermediates in titanium silicate molecular sieves. Adv Catal 48:1–169
Yoshioka H (2006) Oxide ionic conductivity of apatite-type lanthanum silicates. J Alloy Compd 408:649–652
Matteucci F, Dondi M, Guarini G (2002) Effect of soda-lime glass on sintering and technological properties of porcelain stoneware tiles. Ceram Int 28:873–880
Wang W, Blazek K, Cramb A (2008) A study of the crystallization behavior of a new mold flux used in the casting of transformation-induced-plasticity steels. Metall Mater Trans B 39:66–74
Gao H, Yang Y, Huang Q, Wang Q (2017) Pb-Laden CRT glass as classifying hazardous waste definition or exemption in landfill disposal in China. J Mater Cycles Waste Manag 19:241–246
Fedorov AG, Pilon L (2002) Glass foams: formation, transport properties, and heat, mass, and radiation transfer. J Non-Crystal Solid 311:154–173
Fernandes HR, Tulyaganov DU, Ferreira JMF (2009) Preparation and characterization of foams from sheet glass and fly ash using carbonates as foaming agents. Ceram Int 35:229–235
Kim YW, Kim SH, Wang C, Park CB (2003) Fabrication of microcellular ceramics using gaseous carbon dioxide. J Am Ceram Soc 86:2231–2233
Yanagisawa T, Shimizu T, Kuroda K, Kato C (1990) The preparation of alkyltriinethylaininonium–kaneinite complexes and their conversion to microporous materials. Bull Chem Soc Jpn 63:988–992
Sanz-Moral LM, Rueda M, Nieto A, Novak Z, Knez Z, Martín A (2013) Gradual hydrophobic surface functionalization of dry silica aerogels by reaction with silane precursors dissolved in supercritical carbon dioxide. J Supercrit Fluids 84:74–79
Spiekermann G, Matthew SM, Christian S, Sandro J (2012) Vibrational mode frequencies of silica species in SiO2-H2O liquids and gasses from ab initio molecular dynamics. J Chem Phys 136:154–501
Rhee SW, Choi HH, Park HS (2013) Performance evaluation of material separation from spent fluorescent lamps using the thermal end-cutting method. J Mater Cycles Waste Manag 15:503–509
Yan M, Fan Z (2001) Review durability of materials in molten aluminum alloys. J Mater Sci 36:285–295
Spiekermann G, Steele-MacInnis M, Schmidt C, Jahn S (2012) Vibrational mode frequencies of silica species in SiO2-H2O liquids and glasses from ab initio molecular dynamics. J Chem Phys 136:154501–154513
Givianrad MH, Rabani M, Saber-Tehrani S, Aberoomand-Azar P, Sabzevari MH (2013) Preparation and characterization of nanocomposite, silica aerogel, activated carbon and its adsorption properties for Cd (II) ions from aqueous solution. J Saudi Chem Soc 17:329–335
Khan TA, Nazir M, Khan EA (2013) Adsorptive removal of rhodamine B from textile wastewater using water chestnut (Trapa natans L.) peel: adsorption dynamics and kinetic studies. Toxicol Environ Chem 95:919–931
Hameed BH, Ahmad AL, Latiff KNA (2007) Adsorption of basic dye (methylene blue) onto activated carbon prepared from rattan sawdust. Dye Pigment 75:143–149
Iqbal JM, Ashiq MN (2007) Adsorption of dyes from aqueous solutions on activated charcoal. J Hazard Mater 139:57–66
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All the authors declare that there are no known conflicts of interest regarding the publication of this article. The authors confirm that there are no known conflicts of interest associated with this publication and there has been no specific financial support or research grant for this work that could have influenced its outcome.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hussain, Z., Khan, A., Sultan, N. et al. Thermo-chemical conversion of waste glass into non-vitreous porous material for adsorption application. J Mater Cycles Waste Manag 21, 1132–1143 (2019). https://doi.org/10.1007/s10163-019-00864-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-019-00864-y