Abstract
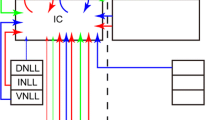
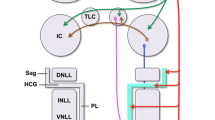
Cholinergic signaling shapes sound processing and plasticity in the inferior colliculus (IC), the midbrain hub of the central auditory system, but how cholinergic terminals contact and influence individual neuron types in the IC remains largely unknown. Using pharmacology and electrophysiology, we recently found that acetylcholine strongly excites VIP neurons, a class of glutamatergic principal neurons in the IC, by activating α3β4* nicotinic acetylcholine receptors (nAChRs). Here, we confirm and extend these results using tissue from mice of both sexes. First, we show that mRNA encoding α3 and β4 nAChR subunits is expressed in many neurons throughout the IC, including most VIP neurons, suggesting that these subunits, which are rare in the brain, are important mediators of cholinergic signaling in the IC. Next, by combining fluorescent labeling of VIP neurons and immunofluorescence against the vesicular acetylcholine transporter (VAChT), we show that individual VIP neurons in the central nucleus of the IC (ICc) are contacted by a large number of cholinergic boutons. Cholinergic boutons were distributed adjacent to the somata and along the full length of the dendritic arbors of VIP neurons, positioning cholinergic signaling to affect synaptic computations arising throughout the somatodendritic compartments of VIP neurons. In addition, cholinergic boutons were occasionally observed in close apposition to dendritic spines on VIP neurons, raising the possibility that cholinergic signaling also modulates presynaptic release onto VIP neurons. Together, these results strengthen the evidence that cholinergic signaling exerts widespread influence on auditory computations performed by VIP neurons and other neurons in the IC.









Similar content being viewed by others
Data Availability
All data will be made available upon reasonable request to the corresponding author.
References
Motts SD, Schofield BR (2009) Sources of cholinergic input to the inferior colliculus. Neuroscience 160(1):103–114. https://doi.org/10.1016/j.neuroscience.2009.02.036
Boucetta S, Cissé Y, Mainville L, Morales M, Jones BE (2014) Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci Off J Soc Neurosci 34(13):4708–4727. https://doi.org/10.1523/JNEUROSCI.2617-13.2014
Gut NK, Winn P (2016) The pedunculopontine tegmental nucleus-A functional hypothesis from the comparative literature. Mov Disord Off J Mov Disord Soc 31(5):615–624. https://doi.org/10.1002/mds.26556
Kroeger D, Ferrari LL, Petit G, Mahoney CE, Fuller PM, Arrigoni E, Scammell TE (2017) Cholinergic, glutamatergic, and GABAergic neurons of the pedunculopontine tegmental nucleus have distinct effects on sleep/wake behavior in mice. J Neurosci Off J Soc Neurosci 37(5):1352–1366. https://doi.org/10.1523/JNEUROSCI.1405-16.2016
Reese NB, Garcia-Rill E, Skinner RD (1995) Auditory input to the pedunculopontine nucleus: I. Evoked potentials Brain Res Bull 37(3):257–264. https://doi.org/10.1016/0361-9230(95)00002-v
Reese NB, Garcia-Rill E, Skinner RD (1995) Auditory input to the pedunculopontine nucleus: II. Unit responses Brain Res Bull 37(3):265–273. https://doi.org/10.1016/0361-9230(95)00001-u
Sakai K (2012) Discharge properties of presumed cholinergic and noncholinergic laterodorsal tegmental neurons related to cortical activation in non-anesthetized mice. Neuroscience 224:172–190. https://doi.org/10.1016/j.neuroscience.2012.08.032
Van Dort CJ, Zachs DP, Kenny JD, Zheng S, Goldblum RR, Gelwan NA, Ramos DM, Nolan MA, Wang K, Weng F-J, Lin Y, Wilson MA, Brown EN (2015) Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep. Proc Natl Acad Sci USA 112(2):584–589. https://doi.org/10.1073/pnas.1423136112
Ayala YA, Malmierca MS (2015) Cholinergic modulation of stimulus-specific adaptation in the inferior colliculus. J Neurosci Off J Soc Neurosci 35(35):12261–12272. https://doi.org/10.1523/JNEUROSCI.0909-15.2015
Askew C, Intskirveli I, Metherate R (2017) Systemic nicotine increases gain and narrows receptive fields in A1 via integrated cortical and subcortical actions. eNeuro 4(3):0192-17. https://doi.org/10.1523/ENEURO.0192-17.2017
Felix RA, Chavez VA, Novicio DM, Morley BJ, Portfors CV (2019) Nicotinic acetylcholine receptor subunit α7-knockout mice exhibit degraded auditory temporal processing. J Neurophysiol 122(2):451–465. https://doi.org/10.1152/jn.00170.2019
Ji W, Gao E, Suga N (2001) Effects of acetylcholine and atropine on plasticity of central auditory neurons caused by conditioning in bats. J Neurophysiol 86(1):211–225. https://doi.org/10.1152/jn.2001.86.1.211
Ji W, Suga N (2009) Tone-specific and nonspecific plasticity of inferior colliculus elicited by pseudo-conditioning: role of acetylcholine and auditory and somatosensory cortices. J Neurophysiol 102(2):941–952. https://doi.org/10.1152/jn.00222.2009
Noftz WA, Beebe NL, Mellott JG, Schofield BR (2020) Cholinergic projections from the pedunculopontine tegmental nucleus contact excitatory and inhibitory neurons in the inferior colliculus. Front Neural Circuits 14:43. https://doi.org/10.3389/fncir.2020.00043
Beebe NL, Schofield BR (2021) Cholinergic boutons are closely associated with excitatory cells and four subtypes of inhibitory cells in the inferior colliculus. J Chem Neuroanat 116:101998. https://doi.org/10.1016/j.jchemneu.2021.101998
Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A (1985) Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci Off J Soc Neurosci 5(5):1307–1315
Cortes R, Probst A, Palacios JM (1984) Quantitative light microscopic autoradiographic localization of cholinergic muscarinic receptors in the human brain: brainstem. Neuroscience 12(4):1003–1026. https://doi.org/10.1016/0306-4522(84)90001-0
Gahring LC, Persiyanov K, Rogers SW (2004) Neuronal and astrocyte expression of nicotinic receptor subunit beta4 in the adult mouse brain. J Comp Neurol 468(3):322–333. https://doi.org/10.1002/cne.10942
Glendenning KK, Baker BN (1988) Neuroanatomical distribution of receptors for three potential inhibitory neurotransmitters in the brainstem auditory nuclei of the cat. J Comp Neurol 275(2):288–308. https://doi.org/10.1002/cne.902750210
Happe HK, Morley BJ (2004) Distribution and postnatal development of alpha 7 nicotinic acetylcholine receptors in the rodent lower auditory brainstem. Brain Res Dev Brain Res 153(1):29–37. https://doi.org/10.1016/j.devbrainres.2004.07.004
Morley BJ, Happe HK (2000) Cholinergic receptors: dual roles in transduction and plasticity. Hear Res 147(1–2):104–112. https://doi.org/10.1016/s0378-5955(00)00124-6
Schwartz RD (1986) Autoradiographic distribution of high affinity muscarinic and nicotinic cholinergic receptors labeled with [3H]acetylcholine in rat brain. Life Sci 38(23):2111–2119. https://doi.org/10.1016/0024-3205(86)90210-9
Sottile SY, Hackett TA, Cai R, Ling L, Llano DA, Caspary DM (2017) Presynaptic neuronal nicotinic receptors differentially shape select inputs to auditory thalamus and are negatively impacted by aging. J Neurosci Off J Soc Neurosci 37(47):11377–11389. https://doi.org/10.1523/JNEUROSCI.1795-17.2017
Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW (1989) Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol 284(2):314–335. https://doi.org/10.1002/cne.902840212
Whiteaker P, Peterson CG, Xu W, McIntosh JM, Paylor R, Beaudet AL, Collins AC, Marks MJ (2002) Involvement of the alpha3 subunit in central nicotinic binding populations. J Neurosci Off J Soc Neurosci 22(7):2522–2529. https://doi.org/10.1523/JNEUROSCI.22-07-02522.2002
Rivera-Perez LM, Kwapiszewski JT, Roberts MT (2021) α3β4 ∗ nicotinic acetylcholine receptors strongly modulate the excitability of VIP neurons in the mouse inferior colliculus. Front Neural Circuits 15:709387. https://doi.org/10.3389/fncir.2021.709387
Beebe NL, Silveira MA, Goyer D, Noftz WA, Roberts MT, Schofield BR (2022) Neurotransmitter phenotype and axonal projection patterns of VIP-expressing neurons in the inferior colliculus. J Chem Neuroanat 102189. https://doi.org/10.1016/j.jchemneu.2022.102189
Goyer D, Silveira MA, George AP, Beebe NL, Edelbrock RM, Malinski PT, Schofield BR, Roberts MT (2019) A novel class of inferior colliculus principal neurons labeled in vasoactive intestinal peptide-Cre mice. eLife 8:e43770. https://doi.org/10.7554/eLife.43770
Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Kvitsani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ (2011) A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71(6):995–1013. https://doi.org/10.1016/j.neuron.2011.07.026
Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13(1):133–140. https://doi.org/10.1038/nn.2467
Kolisnyk B, Al-Onaizi MA, Hirata PHF, Guzman MS, Nikolova S, Barbash S, Soreq H, Bartha R, Prado MAM, Prado VF (2013) Forebrain deletion of the vesicular acetylcholine transporter results in deficits in executive function, metabolic, and RNA splicing abnormalities in the prefrontal cortex. J Neurosci Off J Soc Neurosci 33(37):14908–14920. https://doi.org/10.1523/JNEUROSCI.1933-13.2013
Gillet C, Goyer D, Kurth S, Griebel H, Kuenzel T (2018) Cholinergic innervation of principal neurons in the cochlear nucleus of the Mongolian gerbil. J Comp Neurol 526(10):1647–1661. https://doi.org/10.1002/cne.24433
Goyer D, Kurth S, Gillet C, Keine C, Rübsamen R, Kuenzel T (2016) Slow cholinergic modulation of spike probability in ultra-fast time-coding sensory neurons. eNeuro 3(5). https://doi.org/10.1523/ENEURO.0186-16.2016
Noftz WA, Beebe NL, Mellott JG, Schofield BR (2021) Dense cholinergic projections to auditory and multisensory nuclei of the intercollicular midbrain. Hear Res 411:108352. https://doi.org/10.1016/j.heares.2021.108352
Zhang L, Wu C, Martel DT, West M, Sutton MA, Shore SE (2019) Remodeling of cholinergic input to the hippocampus after noise exposure and tinnitus induction in Guinea pigs. Hippocampus 29(8):669–682. https://doi.org/10.1002/hipo.23058
Choy Buentello D, Bishop DC, Oliver DL (2015) Differential distribution of GABA and glycine terminals in the inferior colliculus of rat and mouse. J Comp Neurol 523(18):2683–2697. https://doi.org/10.1002/cne.23810
Anair JD, Silveira MA, Mirjalili P, Beebe NL, Schofield BR, Roberts MT (2022) Inhibitory NPY neurons provide a large and heterotopic commissural projection in the inferior colliculus. Front Neural Circuits 16:871924. https://doi.org/10.3389/fncir.2022.871924
Marks MJ, Whiteaker P, Collins AC (2006) Deletion of the alpha7, beta2, or beta4 nicotinic receptor subunit genes identifies highly expressed subtypes with relatively low affinity for [3H]epibatidine. Mol Pharmacol 70(3):947–959. https://doi.org/10.1124/mol.106.025338
Marks MJ, Whiteaker P, Grady SR, Picciotto MR, McIntosh JM, Collins AC (2002) Characterization of [(125) I]epibatidine binding and nicotinic agonist-mediated (86) Rb(+) efflux in interpeduncular nucleus and inferior colliculus of beta2 null mutant mice. J Neurochem 81(5):1102–1115. https://doi.org/10.1046/j.1471-4159.2002.00910.x
Salas R, Pieri F, Fung B, Dani JA, De Biasi M (2003) Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci Off J Soc Neurosci 23(15):6255–6263
Smiley JF, Morrell F, Mesulam MM (1997) Cholinergic synapses in human cerebral cortex: an ultrastructural study in serial sections. Exp Neurol 144(2):361–368. https://doi.org/10.1006/exnr.1997.6413
Turrini P, Casu MA, Wong TP, De Koninck Y, Ribeiro-da-Silva A, Cuello AC (2001) Cholinergic nerve terminals establish classical synapses in the rat cerebral cortex: synaptic pattern and age-related atrophy. Neuroscience 105(2):277–285. https://doi.org/10.1016/s0306-4522(01)00172-5
Bieszczad KM, Kant R, Constantinescu CC, Pandey SK, Kawai HD, Metherate R, Weinberger NM, Mukherjee J (2012) Nicotinic acetylcholine receptors in rat forebrain that bind 18F-nifene: relating PET imaging, autoradiography, and behavior. Synap N Y N 66(5):418–434. https://doi.org/10.1002/syn.21530
Zaveri N, Jiang F, Olsen C, Polgar W, Toll L (2010) Novel α3β4 nicotinic acetylcholine receptor-selective ligands. Discovery, structure-activity studies, and pharmacological evaluation. J Med Chem 53(22):8187–8191. https://doi.org/10.1021/jm1006148
Gilmor ML, Nash NR, Roghani A, Edwards RH, Yi H, Hersch SM, Levey AI (1996) Expression of the putative vesicular acetylcholine transporter in rat brain and localization in cholinergic synaptic vesicles. J Neurosci Off J Soc Neurosci 16(7):2179–2190
Weihe E, Tao-Cheng JH, Schäfer MK, Erickson JD, Eiden LE (1996) Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc Natl Acad Sci U S A 93(8):3547–3552. https://doi.org/10.1073/pnas.93.8.3547
Disney AA, Higley MJ (2020) Diverse spatiotemporal scales of cholinergic signaling in the neocortex. J Neurosci Off J Soc Neurosci 40(4):720–725. https://doi.org/10.1523/JNEUROSCI.1306-19.2019
Sarter M, Lustig C (2020) Forebrain cholinergic signaling: wired and phasic, not tonic, and causing behavior. J Neurosci Off J Soc Neurosci 40(4):712–719. https://doi.org/10.1523/JNEUROSCI.1305-19.2019
Krashia P, Moroni M, Broadbent S, Hofmann G, Kracun S, Beato M, Groot-Kormelink PJ, Sivilotti LG (2010) Human α3β4 neuronal nicotinic receptors show different stoichiometry if they are expressed in Xenopus oocytes or mammalian HEK293 cells. PLoS ONE 5(10):e13611. https://doi.org/10.1371/journal.pone.0013611
David R, Ciuraszkiewicz A, Simeone X, Orr-Urtreger A, Papke RL, McIntosh JM, Huck S, Scholze P (2010) Biochemical and functional properties of distinct nicotinic acetylcholine receptors in the superior cervical ganglion of mice with targeted deletions of nAChR subunit genes. Eur J Neurosci 31(6):978–993. https://doi.org/10.1111/j.1460-9568.2010.07133.x
Williams SR, Fletcher LN (2018) A dendritic substrate for the cholinergic control of neocortical output neurons. Neuron. https://doi.org/10.1016/j.neuron.2018.11.035
Mena-Segovia J, Bolam JP (2017) Rethinking the pedunculopontine nucleus: from cellular organization to function. Neuron 94(1):7–18. https://doi.org/10.1016/j.neuron.2017.02.027
Acknowledgements
We thank Nichole Beebe, David Goyer, Jeffrey Mellott, William Noftz, and Brett Schofield for helpful advice and feedback, and Marina Silveira for help with the in situ hybridization experiments.
Funding
This work was supported by National Institutes of Health Grants F31 DC019292 (LMR-P) and R01 DC018284 (MTR).
Author information
Authors and Affiliations
Contributions
All authors conceived of the study, designed experiments, prepared figures, composed and revised the manuscript, and approved the submitted version. JTK and LMR-P performed experiments and analyzed data. MTR obtained funding and supervised the study.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was first published as a preprint: Kwapiszewski JT, Rivera-Perez LM, and Roberts MT (2022). Cholinergic boutons are distributed along the dendrites and somata of VIP neurons in the inferior colliculus. bioRxiv. doi: https://doi.org/10.1101/2022.09.18.508423.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kwapiszewski, J.T., Rivera-Perez, L.M. & Roberts, M.T. Cholinergic Boutons are Distributed Along the Dendrites and Somata of VIP Neurons in the Inferior Colliculus. JARO 24, 181–196 (2023). https://doi.org/10.1007/s10162-022-00885-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-022-00885-9