Abstract
A curative therapy for tinnitus currently does not exist. One may actually exist but cannot currently be causally linked to tinnitus due to the lack of consistency of concepts about the neural correlate of tinnitus. Depending on predictions, these concepts would require either a suppression or enhancement of brain activity or an increase in inhibition or disinhibition. Although procedures with a potential to silence tinnitus may exist, the lack of rationale for their curative success hampers an optimization of therapeutic protocols. We discuss here six candidate contributors to tinnitus that have been suggested by a variety of scientific experts in the field and that were addressed in a virtual panel discussion at the ARO round table in February 2021. In this discussion, several potential tinnitus contributors were considered: (i) inhibitory circuits, (ii) attention, (iii) stress, (iv) unidentified sub-entities, (v) maladaptive information transmission, and (vi) minor cochlear deafferentation. Finally, (vii) some potential therapeutic approaches were discussed. The results of this discussion is reflected here in view of potential blind spots that may still remain and that have been ignored in most tinnitus literature. We strongly suggest to consider the high impact of connecting the controversial findings to unravel the whole complexity of the tinnitus phenomenon; an essential prerequisite for establishing suitable therapeutic approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
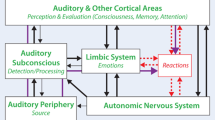
Questions asked by leading scientists in 2020 around the topic ‘tinnitus’ were the focus of a discussion on the 25th of February, 2021, at the Annual Mid-Winter Meeting of the Association for Research in Otolaryngology which was headed by the authors of the present review. The questions spanned topics around the role of (i) inhibitory circuits, (ii) attention, (iii) stress, (iv) sub-entities, (v) development, (vi) perception, and (vii) successful intervention strategies. During the discussion it was suggested that the ongoing research and modular approach to search for the neural correlates of tinnitus resemble the parable of the ‘blind men and the elephant’, the origins of which have been traced to the Indian subcontinent prior to 500 BCE. We here summarize the questions and answers addressed by the panel, sub-divided into seven categories, and point to the possible existence of a blind spot in the field of tinnitus research. The article should not be understood as an all-encompassing review, but as a reference to the respective research interests of the authors of this manuscript in certain areas. Although at the end of this article a single approach is outlined as an apparent "main concept" worthwhile to focus on in an interdisciplinary, international effort, we emphasize that this might possibly hold only for a minority of tinnitus sufferers. The likely existing great variety of tinnitus forms, however, require a great deal of effort to find markers that allow us to better distinguish between the different forms of tinnitus. Within this context we here pinpoint new questions and future tasks for improved tinnitus therapies, and hope to inspire a cohesive motivation to consider viewpoints that were previously less regarded, but when different disciplines collaborate may allow to uncover the neural correlate of tinnitus as a decipherable phenomenon that can ultimately be therapeutically addressed (Fig. 1).
It was six men of Indostan
To learning much inclined,
Who went to see the Elephant
(Though all of them were blind),
That each by observation
Might satisfy his mindFootnote 1
Topic 1: Contribution of Bottom-up and Top-down Inhibitory Circuits to Tinnitus?
The initial discussions centered around the possibility that hearing loss or peripheral deafferentation, as a bottom-up contributor, may be linked to a top-down mechanism which leads to a tinnitus percept. How can a minor change in bottom-up activity due to deafferentation trigger neural gain or the bursting of epileptic firing that, through a possible qualitative shift in GABAergic activity, may in the end lead to a tinnitus percept? Can bottom-up and top-down activity changes induce tinnitus percepts independently, or does bottom-up neural activity trigger a top-down mechanism? There was agreement that a bottom-up mechanism, independent of whether it is caused by hearing loss, cochlear damage, or deafferentation, will be linked to a top-down modulation through the limbic system and attentional circuits. It was suggested that this ‘two-step’ process, likely triggered through cochlear damage and reduced auditory input, alters upstream neurotransmitters such as, GABA and changes signal processing via, for example, stochastic resonance (Krauss et al. 2016); this might change the upstream coding underlying normal perception. On that basis, it is unimportant whether the bottom-up mechanism acts through increased spontaneous activity, increased synchrony, increased bursting, adaptive stochastic resonance or neural gain: the crucial open question would be to understand how the ‘higher-order’ top-down mechanism induces a chronic manifestation of tinnitus over time.
A long-standing discussion of numerous tinnitus models deals with the idea of a two-step bottom-up and top-down mechanism during tinnitus generation, albeit from different perspectives: (i) a bottom-up deprivation that leads to a top-down modulation failure of auditory gating and noise cancellation, or an impaired central gate keeper that leads to a tinnitus percept (Leaver et al. 2011; Rauschecker 2010; Rauschecker et al. 2014), (ii) a bottom-up tinnitus development linked to hearing loss that can lead to a tinnitus percept independently of any top-down mechanism. Here, top-down tinnitus results from a network problem between the auditory and non-auditory brain areas, including the pregenual anterior cingulate cortex (Vanneste et al. 2018a, b) linked to thalamocortical dysrhythmia (Vanneste et al. 2018a, b, 2019), (iii) a bottom-up tinnitus precursor that is normally ignored as imprecise evidence against the prevailing percept of ‘silence’ (Sedley et al. 2016) and that is amplified through a top-down mechanism through focused attention (Hullfish et al. 2019; Sedley et al. 2016), fear, anxiety, or stress (Jastreboff et al. 1996), or through a combination of these facilitators. Influences on the individual tinnitus severity depend on the context of their culture and experience (Searchfield 2014).
Thus, if we ask under which circumstances minor cochlear deafferentation − as a starting point in bottom-up tinnitus development − might change GABAergic strength in affected circuits to such a level that it could profoundly alter top-down circuits such that upstream coding of normal percepts is permanently altered, we may already have the answer: fundamental differences in central processing and sound coding are expected between the approx. 40 % of auditory fibers with lower spontaneous discharge rate and high thresholds (low-SR-AF) and the 60 % of auditory fibers with high spontaneous firing rates and lowest thresholds (high-SR-AF) (Bharadwaj et al. 2015; Liberman 1978). Up to now, the low-SR-AF are assumed to be particularly vulnerable to acoustic overexposure and ageing (Liberman and Kujawa 2017; Wu et al. 2019). This auditory fiber type has been suggested to drive shifts in inhibitory responses through enhanced central neural gain that are possibly involved in tinnitus (Schaette and McAlpine 2011; Shore et al. 2016). Alternatively, a critical diminution of specifically fast (high-SR) auditory fiber processing has been suggested to be causally linked to tinnitus through shifts in tonic inhibitory responses that lead to a loss of central neural gain (Hofmeier et al. 2018, 2021; Knipper et al. 2020; Möhrle et al. 2019; Refat et al. 2021; Rüttiger et al. 2013; Singer et al. 2013).
As such, the community might reconsider the lost function of high-SR, low threshold neurons in defined tinnitus frequency channels as a bottom-up mechanism and as a rationale to explain numerous observations:
-
(i)
Reduced auditory brainstem and cortical responses in tinnitus (Bramhall et al. 2019; Hofmeier et al. 2018; Koops et al. 2020), reduced functional connectivity in the auditory pathway (Boyen et al. 2014; Lanting et al. 2014), and increased latencies in tinnitus (Hofmeier et al. 2018, 2021; Majhi et al. 2019; Milloy et al. 2017; Möhrle et al. 2019) in connection with decreased myelination of the auditory pathway in tinnitus patients (Koops et al. 2021). The latter was observed by fixelFootnote 2-based analysis in which a tinnitus-related atrophy of the left acoustic radiation near the medial geniculate body was the first evidence of a decrease in myelination of the auditory pathway (Koops et al. 2021). This would suggest a more profound peripheral deafferentation of larger-diameter, high-SR auditory fibers that are more robustly myelinated (Bauer et al. 2007).
-
(ii)
Increased epileptic bursting (Jastreboff et al. 1996), adaptive stochastic resonance (Krauss et al. 2016, 2017, 2018b), and excessive neuronal synchrony in the auditory cortex in tinnitus (Eggermont and Tass 2015; Noreña and Farley 2013) may all be explained through a loss of tonic inhibition that would result in a rapid increase in bursting or epileptic firing and reduced signal-to-noise ratios (Duguid et al. 2012; Hsieh et al. 2017; Rossignol et al. 2013).
-
(iii)
Diminished activity of the tonic fast-spiking parvalbumin (PV)+ interneuron networks would, in turn, be expected to be linked to enhanced baseline spontaneous gamma power (Mamashli et al. 2017), and thus explain enhanced spontaneous gamma oscillations in tinnitus patients (Ortmann et al. 2011; Vanneste et al. 2018b, 2019; Weisz et al. 2007). This would also be compatible with enhanced variance in stimulus-induced responses and lower signal-to-noise ratios that contribute to tinnitus (Zeng 2020) thereby reducing the gate keeping or noise cancellation linked to dysrhythmia (Leaver et al. 2011; Rauschecker 2010; Rauschecker et al. 2014; Vanneste et al. 2019), reducing functional connectivity in the auditory pathway (Boyen et al. 2014; Lanting et al. 2014) (for a review see (Knipper et al. 2020)).
-
(iv)
Increased susceptibility to a clinical manifestation of tinnitus linked to enhanced tinnitus-related distress, as observed in patients with brain-derived neurotrophic factor (BDNF) Val66 Met polymorphism (Vanneste et al. 2021). This single-nucleotide polymorphism (SNP) in the BDNF gene (substitution of valine to methionine) leads to a decrease of activity-dependent intracellular trafficking and secretion of the neuronal BDNF (Chen et al. 2004) which is required for balanced hypothalamus-pituitary-adrenal (HPA) axis control (Jeanneteau et al. 2019). During enhanced memory-linked auditory adjustment processes, fast (high-SR) auditory processing is predicted to drive activity-dependent BDNF translation and secretion in the hippocampus (Eckert et al. 2021; Marchetta et al. 2020; Matt et al. 2018).
-
(v)
The lower risk of tinnitus in congenital deafness (Eggermont and Kral 2016) and in congenital unilateral deafness (Lee et al. 2017), and the elevated risk of tinnitus in the implanted ear of bilaterally or unilaterally deaf children when CI are switched off (Baguley and Atlas 2007; Chadha et al. 2009; Ramakers et al. 2015) (for a review, see (Knipper et al. 2020)).
-
(vi)
Finally, the significantly higher gray matter volume in the lingual gyri observed in hearing loss in the tinnitus group compared to the hearing loss group without tinnitus (Koops et al. 2021), can also be taken as an indicator of the loss of fast auditory processing in tinnitus. The lingual gyrus, also known as the medial occipito-temporal gyrus, is linked preferentially to processing vision (Kozlovskiy et al. 2014). This observation is particularly exciting, as it may point to a loss of clustering of auditory and visual modalities in tinnitus, and provide a rationale for the exuberant connections between the visual and auditory cortex found, for example, in deaf cats, together with an increased visual responsiveness in the auditory cortex (Land et al. 2016). It may support the view that within the frequency channel of the tinnitus percept, the mature, fine-grained wiring that is based on fast (tonic) inhibitory circuits which develops with auditory experience is reversed towards an immature pattern (Knipper et al. 2020). This concept should lead to reconsideration of reported tinnitus-related increases in the connectivity of the left lingual gyrus with the left auditory cortex (Hinkley et al. 2015) and decreased connectivity of the left lingual gyrus with the auditory resting-state network (Schmidt et al. 2013, 2017).
Future Tasks to See and Test for a Possible Blind Spot
The contribution of fast (high-SR) auditory fiber dysfunction as the trigger of a bottom-up mechanism that, through altered tonic inhibitory strength, leads to top-down activity changes that promote the development of a tinnitus percept could be tested in future studies. A possible approach would be the use of pharmaceutical drugs that either antagonize tonic or phasic GABA receptor units in behavioral animal models of tinnitus, followed by clinical trials. Additionally, morphometric measurements of auditory nerve density could enable to examine the contributions of large-diameter high-SR auditory nerve fibers in tinnitus patients. Moreover, such contributions could then be analysed for associations with changes in top-down cortical oscillatory signatures and dysrhythmia (Lee et al. 2020; van Gendt et al. 2012) or distress and functional-network topology changes, as described in the context of tinnitus (Yoo et al. 2021).
Topic 2: Contribution of Attention to Tinnitus and Its Relation to Long-term Habituation and Resilience
There are numerous studies that report impaired or altered executive attention, selective attention, and working memory in chronic tinnitus (Khan and Husain 2020; Mazurek et al. 2019; Mohamad et al. 2016; Nagaraj et al. 2020). Moreover, no doubt exists that the distress accompanying tinnitus influences general and crystallized intelligence and executive function (Mohamad et al. 2016; Neff et al. 2021). Tinnitus appears to be a symptom of a hyperactive cognitive control network: In this maladaptive network, the negative emotion experienced during tinnitus leads to a consumption of cognitive resources that are typically required for proper functioning of auditory working memory. This imbalanced cognitive control contributes to increased vigilance to the tinnitus tone (Mazurek et al. 2019; Trevis et al. 2016). This concept was confirmed, by previous studies of (Brozoski et al. 2019), who observed that in rats, behaviorally-evidenced tinnitus promotes an increased vigilance to the tinnitus percept, while attention to an auditory-specific task is diminished. In this connection, long-term habituation during tinnitus might be associated with a gradual decline of such ‘negative emotions’, or with a decline of factors that contribute to these negative emotions (Elarbed et al. 2021; Luan et al. 2019; Mazurek et al. 2019; Nagaraj et al. 2020).
The contribution of cognitive and perceptual load should also be discussed in this context (Khan and Husain 2020). Perceptual load-tinnitus is an undesired stimulation of the auditory processing pathway, and constant perception of tinnitus affects the cognitive load. Within the same framework, the possible causes of tinnitus resilience could be the same as those that contribute to habituation to tinnitus. In general, resilience is the ability to cope with critical situations through the use of personal and socially mediated resources. Thus, factors for resilience are positive emotions, socio-environmental factors, cognitive flexibility, active coping style, and exercise (Faye et al. 2018). Negative or positive environmental or hereditary conditions, differences in early life-stress, or chronic illness leading to depression or anxiety, and differences in temperament or personality, contribute to the so-called ‘emotional health’ that influences tinnitus and span degrees from complete tinnitus resilience to a high risk for tinnitus (Aydin and Searchfield 2019; Marks et al. 2019; Searchfield 2014; Searchfield et al. 2020). On the clinical side, high resilience in tinnitus patients is associated with less depression, less anxiety and fewer somatic symptoms, and the relationship between resilience and tinnitus distress is mediated by emotional health (Wallhäusser-Franke et al. 2014). In this sense, the lack of tinnitus habituation could be explained as a result of a reduced resilience strategy. Here, for example, the absence of hereditary contributors to enhanced distress, which have been shown to increase susceptibility to tinnitus (Amanat et al. 2020; Lopez-Escamez and Amanat 2020; Ruan et al. 2018; Szczepek et al. 2019), including the BDNF Val 66 Met polymorphism (Vanneste et al. 2021), have to be regarded in the context of a possible contribution to tinnitus resilience and long-term habituation.
Future Tasks to See and Test for a Possible Blind Spot
The community of tinnitus researchers may reconsider how individual environmental or hereditary differences in attentional/cognitive brain states could contribute to suffering more or less from enhanced ‘noise’ in tinnitus frequency channels that have lost contrast-amplification after reduced fast (high-SR) auditory processing. The strong relation of fast (high-SR) auditory processing as a bottom-up mechanism with attentional/cognitive brain states (Review (Knipper et al. 2020)) needs urgent examination. As additional factors that might directly contribute to hereditary or environmental differences in individuals’ and thereby contribute to either enhanced or decreased habituation or resilience to tinnitus, we suggest to consider also those factors that promote the metabolic fatigue of fast auditory processing. This would include conditions that diminish the function of fast PV + interneurons (PV + IN) activity (Kann et al. 2016), which require a high action-potential (AP)-related energy budget to maintain high-frequency activity and fast temporally precise transmission (Hu et al. 2018).
Topic 3: Contribution of Stress to Tinnitus and Its Link to the Limbic System and Environmental Factors
The list of studies that demonstrate a close relationship of distress and tinnitus is increasing (Boecking et al. 2021; Durai et al. 2018; Elarbed et al. 2021; Park et al. 2020; van Munster et al. 2020). Numerous studies document altered evoked or resting state BOLD fMRI or EEG activity in tinnitus patients, particularly in those brain regions or networks that are involved in attention, distress, and memory functions (Kandeepan et al. 2019; Mohsen et al. 2019; Pattyn et al. 2016; Vanneste et al. 2021; Yoo et al. 2021). The strong contribution of distress culminates in evidence that insomnia, hearing distress, and anxiety are the best predictors of tinnitus severity, and are indeed stronger predictors than any demographic factors (Beukes et al. 2021; Crönlein et al. 2016).
But what do we already know about the mechanism by which distress influences tinnitus? It is important to distinguish the contribution of oxidative stress, stressful acoustic trauma, or mental stress, which has not yet been considered adequately. All forms of stressful events may eventually accumulate to an imbalance within the HPA axis, leading to differential activation of glucocorticoid and mineralocorticoid receptors, and thereby contribute to chronic tinnitus (Kraus and Canlon 2012; Mazurek et al. 2012; Simoens and Hébert 2012). Enhanced tinnitus-related distress, as observed in patient groups with BDNF Val66 Met polymorphism (Vanneste et al. 2018a) would be compatible with BDNF bridging glucocorticoid effects on brain networks through BDNF driven phosphorylation of glucocorticoid receptor (Jeanneteau et al. 2019). Impaired glucocorticoid receptor (GR) phosphorylation following a reduced activity-dependent BDNF recruitment has been shown to lead to impaired long-term memory retention and deficits in forming postsynaptic dendritic spines after, for example, motor-skill training (Arango-Lievano et al. 2019). Thus it is conceivable that, under healthy conditions, fast (high-SR) auditory fiber processing may recruit activity-dependent BDNF to energize contrast amplification and distress levels (Knipper et al. 2020; Matt et al. 2018).
Currently we do not have a good explanation for the potential differential – or combined − impact that acoustic trauma and stress have on different tinnitus groups, which can be clearly distinguished by their distress response. The analysis of 1228 patients led to four distinct patient phenotypes: divided into (i) an ‘avoidant group’, with few affective or psychosomatic symptoms and less tinnitus distress, (ii) a ‘psychosomatic group´ with profound psychosomatic, emotional, and somatic burden, risk of depression and anxiety, and reduced quality of life, (iii) a ‘somatic group’ with physical symptoms that create distress or underlying medical conditions and higher somatic complaints, like pain or headache, and (iv) a ‘distress group’ with a high level of passive stress and physical exhaustion, anxious depressed mood, these patients are often younger and with correlations of neuroticism and anxiety (Bartels et al. 2010; Niemann et al. 2020).
Future Tasks to See and Test for a Possible Blind Spot
We need to understand how bottom-up mechanisms may be linked to the role that distress plays in tinnitus − which has been judged to be the ‘best predictor of tinnitus severity’ (Beukes et al. 2021; Crönlein et al. 2016). Here, BDNF signaling needs to be considered as a potential bridge linking bottom-up changes in tinnitus with an altered distress network. Deficits in BDNF driven GR phosphorylation and LTP retention should be considered in the context of enhanced tinnitus-related distress in patients suffering from the BDNF Val66Met polymorphism (Vanneste et al. 2021).
Topic 4: Contribution of Non-identified Sub-entities of Tinnitus to Current Controversial Views on the Neural Correlate of Tinnitus
Previous studies analyzing patients with tinnitus and hyperacusis (T+H group) and patients without hyperacusis (T-group) revealed evidence for the existence of tinnitus subcategories (Hofmeier et al. 2018, 2021). When tinnitus co-occurred with hyperacusis, stimuli-evoked increases in sound-evoked, supra-threshold ABR amplitudes and fMRI BOLD responses in the medial geniculate body (MGB) as well as in the auditory cortex were found (Hofmeier et al. 2021; Koops and van Dijk 2021; Refat et al. 2021). While a higher sound-evoked fMRI BOLD activity in cortical and subcortical auditory structures was observed in the tinnitus group with hyperacusis compared to the tinnitus group without hyperacusis, an enhanced response to sound was not found in the tinnitus frequency regions (Koops and van Dijk 2021). On the other hand, in tinnitus patients without hyperacusis the auditory responsiveness, including spontaneous and evoked fMRI BOLD responses was reduced (Hofmeier et al. 2021; Refat et al. 2021). Not only the annoyance, tinnitus loudness and bilateral tinnitus are higher in the T+H group (Hofmeier et al. 2021; Ralli et al. 2017; Refat et al. 2021; Schecklmann et al. 2014), but the co-occurrence of tinnitus with hyperacusis also leads to an increase of tinnitus duration over time (Refat et al. 2021; Vielsmeier et al. 2020). Moreover, when chirp stimuli of different frequency spectra were employed (Hofmeier et al. 2021), the results pointed to reduced auditory response activity in T-groups to higher-frequency stimuli and to enhanced auditory response patterns to lower-frequency stimuli in T+H groups (Hofmeier et al. 2021). The more widespread signal amplification process in patients with tinnitus and hyperacusis is supposed to proceed through an excessive thalamo-cortical activity that may trigger an excitation spread to limbic and pain regions, and ultimately results in an over-attention to increased loudness at all sound frequencies (Hofmeier et al. 2021; Koops et al. 2020). It is conceivable that the enhanced distress levels in T+H patients (Hofmeier et al. 2021; Ralli et al. 2017; Refat et al. 2021; Schecklmann et al. 2014) affect wider frequency ranges, including the pain network (Hofmeier et al. 2021; Refat et al. 2021) and contribute directly to the enhanced annoyance to the ‘noise’ in higher-frequency tinnitus channels.
Overall, the hypothesis that sub-entities of tinnitus exist, and can possibly co-occur in different frequency channels, needs to be validated in larger cohort groups.
We may speculate that even controversial findings of deficits of speech-in-noise intelligibility of tinnitus patients may have their origin in different prevalence of sub-entities of tinnitus. Accordingly, a study of (Zeng 2020) did not find any differences in speech-in-noise intelligibility using a group of young adults (mean age 22.6 years) as a normal-hearing control compared to a heterogeneous group of 45 adults (mean age 44 years) with chronic tinnitus (Zeng 2020). In contrast, (Bureš et al. 2019), who found a poorer ability to detect tones in noise and a higher sensitivity to intensity changes and interaural time differences, used a more harmonized group of 51 tinnitus subjects aged around 66 years, and 68 controls around 69 years. It is feasible that a difference in the prevalence of patients with T+H co-occurrence between both studies explain the difference in speech-in-noise intelligibility between the groups.
Future Tasks to See and Test a Possible Blind Spot
The current evidence suggests that hyperacusis and tinnitus pathologies may co-exist in parallel frequency channels of the bottom-up auditory pathways. There is an urgent need to consider that in patients with tinnitus and hyperacusis parallel bottom-up changes exist that differentially affect top-down circuits (Eggermont 2021; Martel and Shore 2020) this hypothesis needs to be validated in larger cohort groups and through multi-center clinical trials.
Topic 5: Contribution of Maladaptive Information Transmission to Tinnitus?
This question may need to be considered in the context of the brain’s overall ability to constantly try to optimize information transmission from the periphery into the brain (Krauss et al. 2016, 2017, 2018a). In the various disciplines in neuroscience, the optimization of information transmission from the periphery into the brain can be (i) realized through stochastic resonance, where added neuronal noise lifts spontaneous firing rate (SFR) above the threshold, causing a sensory percept (Krauss et al. 2016; Schilling et al. 2021; White et al. 2019). (ii) Alternatively, based on the Bayesian model, the brain is conceived as a prediction machine that informs its memory-based predictions through sensory updating (Hemmer et al. 2015). In this view, tinnitus is the result of a prediction error between the predicted and the actual auditory input. The decrease in sensory updating is reflected by decreased alpha activity, while the prediction error is believed to result in altered theta-gamma and beta-gamma coupling (De Ridder et al. 2015; Durai et al. 2019; Hullfish et al. 2019; Mohebbi et al. 2019). Both models may be covered in another (iii) view that the information transfer from the periphery into the brain operates in a so-called ‘reverberating regime,’ (Cramer et al. 2020; Wilting et al. 2018). This describes an information process that, for example, enables cortical networks to interpolate between the asynchronous-irregular and the critical state by small changes in effective synaptic strength or by the excitation-inhibition ratio (Cramer et al. 2020; Wilting et al. 2018).
In these information processing models, we may miss determinants that define (i) the thresholds for action potentials lowered by stochastic resonance, (ii) the set point or threshold to which predictions are typically made (memory or detection threshold?), (iii) the baseline at which the system reverberates.
Future Tasks to See and test a Potential Blind Spot
Considering tinnitus as a diminution of fast (high-SR fiber dependent) auditory processing (Knipper et al. 2020; Zeng 2020), the baseline signal-to-noise ratio would be lost in affected frequency channels. In this view, the brain’s advantage having tinnitus in deprived frequency channels would be to lower the energy budget of the brain. The reversal from a mature fast-processing circuit to a presumably immature state within the affected frequency channels would prevent further metabolic fatigue. Thus, fast inhibitory PV+ interneurons and fast (high-SR) auditory processing (Knipper et al. 2020), comprise of only 2.6–4.6 % of interneurons - but require 14–25 % of the total AP related energy budget of the brain (Hu et al. 2014, 2018). This might explain the high vulnerability for metabolic fatigue of PV+ interneurons (Kann et al. 2016). The evolutionary gain in separately clustering sensory modalities to enhance fast information processing and sensory-specific acuity may create a risk of losing it. On a fast time scale, a mechanism like stochastic resonance, which constantly optimizes information transmission from the periphery to the brain, would improve auditory processing at the cost of generating a tinnitus percept. In that view, tinnitus would be a side effect of the brain’s effort to improve hearing (Gollnast et al. 2017).
Topic 6: Contribution of Deafferentation to a Tinnitus Percept?
It is still unclear how, through altered GABAergic circuit strength or differential tonic or phasic GABA receptor activation, a bottom-up activity might lead to a tinnitus percept? Bottom-up activity must change activity spreading from the thalamus through a canonical propagation to the different cortical layers, from layer IV to supra-granular layer I–II, and subsequently through a top-down mechanism from the infra-granular layer V/VI to the limbic system (Norena et al. 2021), finally leading to a conscious sound percept. Here, the Bayesian models, including predictive coding, attentional modulation and cortical oscillatory band activity, as neurophysiological substrates for auditory predictions, were suggested to contribute through incomplete top-down processing during auditory scene analysis to the conscious tinnitus percept (Durai et al. 2018; Hullfish et al. 2018). It has been speculated that during the perception of tinnitus, spatio-temporal activity patterns in the auditory cortex must be specific to the quality of the percept, and different from patterns that may be recorded during silence but without any phantom percept (Krauss et al. 2018b).
The discussion included the idea that in a chronically perceived tinnitus percept, minor undetectable changes may also contribute, and propagate, possibly as an adaptive response to a transient signal that has occurred only for a few milliseconds within the bottom-up path. In an attempt to illustrate the scenario of a host of existing tinnitus researchers currently hypothesizing several hundreds of different tinnitus decisive factors, the example of "the Blind men and the Elephant" was brought up:
Blind to see the coherent whole behind a tinnitus percept, being too fixated on one individual part.
Future Tasks to See and test for a Possible Blind Spot
Here, we dare to suggest one factor that the tinnitus community may still be too ‘blind’ to, and that could explain most of the different existing tinnitus models and theories, to wit: the observation that the conscious percept of an auditory stimulus requires a proper maturation of a baseline. On top of this baseline, the auditory stimuli can be facilitated via integration into a fronto-striatal contrast amplification circuit to lead to a percept (Irvine 2018; Oxenham 2018). When we assume that the baseline is lost in ascending bottom-up circuits in distinct frequency channels (Knipper et al. 2020), the top-down circuit will now amplify the ‘enhanced noise’ that is generated as a result of lost contrast amplification in the affected region. The intensity of the ‘amplification process’ may, in turn, heavily depend on the individual’s ‘emotional stage’.
Topic 7: Future Therapy Approaches on the Basis of Ongoing Controversies About the Neural Correlate of Tinnitus
The strong contribution of distress as the best predictor of tinnitus severity (Beukes et al. 2021; Crönlein et al. 2016) may explain why cognitive behavioral therapy (CBT) has the highest effectiveness in the therapy of chronic tinnitus (European Guideline) (Aazh et al. 2019; Cima et al. 2019). The numerous studies that report stress as a co-factor for tinnitus (Brüggemann et al. 2017; Knopke et al. 2017; Ramakers et al. 2015) justify cognitive behavioral therapy as the currently most effective therapy with the best reduction of tinnitus burden.
Besides the gold standard of rather unspecific CBT, which has been propagated for many years, the concept of bimodal neuromodulation has currently gained attention in tinnitus research through various studies that apparently provide surprisingly good results (Conlon et al. 2020; Riffle et al. 2020). Regarding the contributing factor of general health on tinnitus, efforts to reduce tinnitus by diet (Spankovich and Le Prell 2019) or physical activity (Carpenter-Thompson et al. 2015; Michiels et al. 2016) need further thought. The benefit of invasive tinnitus treatment may outweigh its risks, but with one exception: the extraordinarily invasive method of cochlear implantation. Indeed, increasing evidence suggests that tinnitus can be silenced in most of the implanted tinnitus patients with deafness or severe hearing loss (Baguley and Atlas 2007; Kleine Punte et al. 2013; Knopke et al. 2017; Li et al. 2019; Mertens et al. 2018; Pillsbury et al. 2018; Tyler et al. 2008). This would, of course, still not justify cochlear implantation in tinnitus patients that have little or no hearing loss. A brainstem auditory implant would possibly be beneficial in such cases (Van Den Berge et al. 2019).
The potential of vagus nerve stimulation (VNS) to drive neural plasticity to reduce or eliminate the neural drivers of ongoing tinnitus, although recently judged as a successful approach (De Ridder et al. 2020), may have too many side effects. Effects of pairing of the vagus stimulation with non-tinnitus or tinnitus-matched sounds still has to be determined (De Ridder et al. 2020).
Despite their potential to silence intermittent tinnitus under distinct conditions, other therapies, including, for example, lidocaine, (Vielsmeier et al. 2021), may have too severe side effects. These include psychotropic effects, or effects on blood pressure (Tran and Koo 2014), and their benefits do not seem to outweigh their risks.
Future Tasks to See and Test for a Possible Blind Spot
As noted previously, it may be conceivable to work on strategies that specifically trigger stochastic resonance. Alternatively, acoustic stimulation may be re-considered, consistent with previous findings that reported the suppression or relief of the tinnitus percept by electric-acoustic stimulation or by hearing aids (Kleine Punte et al. 2013; Knopke et al. 2017; Li et al. 2019; Mertens et al. 2018; Peters et al. 2020; Pillsbury et al. 2018; Searchfield et al. 2010; Shekhawat et al. 2013).
CONCLUSION
We suggest that the tinnitus percept resulting from the amplification of ‘enhanced noise’ that is generated as a result of lost contrast amplification subsequent to a loss of fast (high-SR) auditory processing is compatible with numerous apparently controversial findings. In this view certain forms of tinnitus may result from loss of fast (high-SR) auditory fibers and subsequent diminished capacity to suppress intrinsic noise levels in affected frequency channels through PV-IN driven fronto-striatal contrast amplification circuits. This signal is required to energize gate keeping, contrast amplification, neural gain, and adjustments, but when reduced as a result of diminished fast (high-SR) auditory fiber processing, would entail dysfunctions in affected frequency channels that themselves result in elevated noise levels and tinnitus percept. Tinnitus percept is amplified through increased vigilance to the noise following imbalanced cognitive control and lost contrast amplification. Tinnitus percept is amplified through increased vigilance to the noise following imbalanced cognitive control and lost contrast amplification. The tinnitus loudness and burden may differ in individuals, depending on their individual ‘emotional stage’ and distress level. Important to emphasize that this viewpoint may hold only for a small minority of tinnitus sufferers, regarding that likely a great variety of tinnitus forms exists. The authors are however convinced that the search for the most comprehensive possible concrete therapy forms is doomed to failure at least as long as we do not succeed in enabling a better clinical characterization of single forms of tinnitus in patients. In a unified effort across the tinnitus community, tinnitus research could focus on examination of this suggested concept, and find new curative approaches to silence tinnitus.
Notes
Saxe, John Godfrey. "The Blind Men and the Elephant". The poems of John Godfrey Saxe. p. 260.
Fixel refers to a specific fibre bundle within a specific voxel.
References
Aazh H, Landgrebe M, Danesh AA, Moore BC (2019) Cognitive behavioral therapy for alleviating the distress caused by tinnitus, hyperacusis and misophonia: current perspectives. Psychol Res Behav Manag 12:991–1002. https://doi.org/10.2147/PRBM.S179138
Amanat S, Gallego-Martinez A, Lopez-Escamez JA (2020) Genetic inheritance and its contribution to tinnitus. Curr Top Behav Neurosci. https://doi.org/10.1007/7854_2020_155
Arango-Lievano M, Borie AM, Dromard Y, Murat M, Desarmenien MG, Garabedian MJ, Jeanneteau F (2019) Persistence of learning-induced synapses depends on neurotrophic priming of glucocorticoid receptors. Proc Natl Acad Sci USA 116(26):13097–13106. https://doi.org/10.1073/pnas.1903203116
Aydin N, Searchfield GD (2019) Changes in tinnitus and physiological biomarkers of stress in response to short-term broadband noise and sounds of nature. Complement Ther Med 46:62–68. https://doi.org/10.1016/j.ctim.2019.07.018
Baguley DM, Atlas MD (2007) Cochlear implants and tinnitus. Prog Brain Res 166:347–355. https://doi.org/10.1016/S0079-6123(07)66033-6
Bartels H, Middel B, Pedersen SS, Staal MJ, Albers FWJ (2010) The distressed (type D) personality is independently associated with tinnitus: a case–control study. Psychosomatics 51(1):29–38. https://doi.org/10.1176/appi.psy.51.1.29
Bauer CS, Woolley RJ, Teschemacher AG, Seward EP (2007) Potentiation of exocytosis by phospholipase C-coupled G-protein-coupled receptors requires the priming protein Munc13-1. J Neurosci 27(1):212–219. https://doi.org/10.1523/JNEUROSCI.4201-06.2007
Beukes EW, Manchaiah V, Allen PM, Andersson G, Baguley DM (2021) Exploring tinnitus heterogeneity. Prog Brain Res 260:79–99. https://doi.org/10.1016/bs.pbr.2020.05.022
Bharadwaj HM, Masud S, Mehraei G, Verhulst S, Shinn-Cunningham BG (2015) Individual differences reveal correlates of hidden hearing deficits. J Neurosci 35(5):2161–2172. https://doi.org/10.1523/JNEUROSCI.3915-14.2015
Boecking B, Rose M, Brueggemann P, Mazurek B (2021) Two birds with one stone—addressing depressive symptoms, emotional tension and worry improves tinnitus-related distress and affective pain perceptions in patients with chronic tinnitus. PLoS One. https://doi.org/10.1371/journal.pone.0246747
Boyen K, de Kleine E, van Dijk P, Langers DRM (2014) Tinnitus-related dissociation between cortical and subcortical neural activity in humans with mild to moderate sensorineural hearing loss. Hear Res 312:48–59. https://doi.org/10.1016/j.heares.2014.03.001
Bramhall NF, McMillan GP, Gallun FJ, Konrad-Martin D (2019) Auditory brainstem response demonstrates that reduced peripheral auditory input is associated with self-report of tinnitus. J Acoust Soc Am 146(5):3849–3862. https://doi.org/10.1121/1.5132708
Brozoski T, Wisner K, Randall M, Caspary D (2019) Chronic sound-induced tinnitus and auditory attention in animals. Neuroscience 407:200–212. https://doi.org/10.1016/j.neuroscience.2018.10.013
Brüggemann P, Szczepek AJ, Klee K, Gräbel S, Mazurek B, Olze H (2017) In patients undergoing cochlear implantation, psychological burden affects tinnitus and the overall outcome of auditory rehabilitation. Front Hum Neurosci. https://doi.org/10.3389/fnhum.2017.00226
Bureš Z, Profant O, Svobodová V, Tóthová D, Vencovský V, Syka J (2019) Speech comprehension and its relation to other auditory parameters in elderly patients with tinnitus. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2019.00219
Carpenter-Thompson JR, McAuley E, Husain FT (2015) Physical activity, tinnitus severity, and improved quality of life. Ear Hear 36(5):574–581. https://doi.org/10.1097/AUD.0000000000000169
Chadha NK, Gordon KA, James AL, Papsin BC (2009) Tinnitus is prevalent in children with cochlear implants. Int J Pediatr Otorhinolaryngol 73(5):671–675. https://doi.org/10.1016/j.ijporl.2008.12.032
Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS (2004) Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci 24(18):4401–4411. https://doi.org/10.1523/JNEUROSCI.0348-04.2004
Cima RFF, Mazurek B, Haider H, Kikidis D, Lapira A, Norena A, Hoare DJ (2019) A multidisciplinary European guideline for tinnitus: diagnostics, assessment, and treatment. HNO 67(1):10–42. https://doi.org/10.1007/s00106-019-0633-7
Conlon B, Langguth B, Hamilton C, Hughes S, Meade E, Connor CO, Schecklmann M, Hall DA, Vanneste S, Leong SL, Subramaniam T, D’Arcy S, Lim HH (2020) Bimodal neuromodulation combining sound and tongue stimulation reduces tinnitus symptoms in a large randomized clinical study. Sci Transl Med. https://doi.org/10.1126/scitranslmed.abb2830
Cramer B, Stöckel D, Kreft M, Wibral M, Schemmel J, Meier K, Priesemann V (2020) Control of criticality and computation in spiking neuromorphic networks with plasticity. Nat Commun. https://doi.org/10.1038/s41467-020-16548-3
Crönlein T, Langguth B, Pregler M, Kreuzer PM, Wetter TC, Schecklmann M (2016) Insomnia in patients with chronic tinnitus: cognitive and emotional distress as moderator variables. J Psychosom Res 83:65–68. https://doi.org/10.1016/j.jpsychores.2016.03.001
De Ridder D, Vanneste S, Langguth B, Llinas R (2015) Thalamocortical dysrhythmia: a theoretical update in tinnitus. Front Neurol. https://doi.org/10.3389/fneur.2015.00124
De Ridder D, Langguth B, Vanneste S (2020) Vagus nerve stimulation for tinnitus: a review and perspective. Prog Brain Res. https://doi.org/10.1016/bs.pbr.2020.08.011
Duguid I, Branco T, London M, Chadderton P, Häusser M (2012) Tonic inhibition enhances fidelity of sensory information transmission in the cerebellar cortex. J Neurosci 32(32):11132–11143. https://doi.org/10.1523/JNEUROSCI.0460-12.2012
Durai M, O’Keeffe MG, Searchfield GD (2018) A review of auditory prediction and its potential role in tinnitus perception. J Am Acad Audiol 29(6):533–547. https://doi.org/10.3766/jaaa.17025
Durai M, Sanders M, Kobayashi K, Searchfield GD (2019) Auditory streaming and prediction in tinnitus sufferers. Ear Hear 40(2):345–357. https://doi.org/10.1097/AUD.0000000000000620
Eckert P, Marchetta P, Manthey MK, Walter MH, Jovanovic S, Savitska D, Singer W, Jacob MH, Rüttiger L, Schimmang T, Milenkovic I, Pilz PKD, Knipper M (2021) Deletion of BDNF in Pax2 lineage-derived interneuron precursors in the hindbrain hampers the proportion of excitation/inhibition, learning, and behavior. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2021.642679
Eggermont JJ (2021) Separate auditory pathways for the induction and maintenance of tinnitus and hyperacusis? Prog Brain Res 260:101–127. https://doi.org/10.1016/bs.pbr.2020.01.006
Eggermont JJ, Tass PA (2015) Maladaptive neural synchrony in tinnitus: origin and restoration. Front Neurol. https://doi.org/10.3389/fneur.2015.00029
Eggermont JJ, Kral A (2016) Somatic memory and gain increase as preconditions for tinnitus: insights from congenital deafness. Hear Res 333:37–48. https://doi.org/10.1016/j.heares.2015.12.018
Elarbed A, Fackrell K, Baguley DM, Hoare DJ (2021) Tinnitus and stress in adults: a scoping review. Int J Audiol 60(3):171–182. https://doi.org/10.1080/14992027.2020.1827306
Faye C, Mcgowan JC, Denny CA, David DJ (2018) Neurobiological mechanisms of stress resilience and implications for the aged population. Curr Neuropharmacol 16(3):234–270. https://doi.org/10.2174/1570159x15666170818095105
Gollnast D, Tziridis K, Krauss P, Schilling A, Hoppe U, Schulze H (2017) Analysis of audiometric differences of patients with and without tinnitus in a large clinical database. Front Neurol. https://doi.org/10.3389/fneur.2017.00031
Hemmer P, Tauber S, Steyvers M (2015) Moving beyond qualitative evaluations of Bayesian models of cognition. Psychon Bull Rev 22(3):614–628. https://doi.org/10.3758/s13423-014-0725-z
Hinkley LB, Mizuiri D, Hong O, Nagarajan SS, Cheung SW (2015) Increased striatal functional connectivity with auditory cortex in tinnitus. Front Hum Neurosci 9(OCTOBER):568. https://doi.org/10.3389/fnhum.2015.00568
Hofmeier B, Wolpert S, Aldamer ES, Walter M, Thiericke J, Braun C, Zelle D, Rüttiger L, Klose U, Knipper M (2018) Reduced sound-evoked and resting-state BOLD fMRI connectivity in tinnitus. NeuroImage Clin 20:637–649. https://doi.org/10.1016/j.nicl.2018.08.029
Hofmeier B, Wertz J, Refat F, Hinrichs P, Saemisch J, Singer W, Rüttiger L, Klose U, Knipper M, Wolpert S (2021) Functional biomarkers that distinguish between tinnitus with and without hyperacusis. Clin Transl Med 11(5):e378. https://doi.org/10.1002/ctm2.378
Hsieh TH, Cheong Lee HH, Hameed MQ, Pascual-Leone A, Hensch TK, Rotenberg A (2017) Trajectory of parvalbumin cell impairment and loss of cortical inhibition in traumatic brain injury. Cereb Cortex 27(12):5509–5524. https://doi.org/10.1093/cercor/bhw318
Hu H, Gan J, Jonas P (2014) Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science. https://doi.org/10.1126/science.1255263
Hu H, Roth FC, Vandael D, Jonas P (2018) Complementary tuning of Na+ and K+ channel gating underlies fast and energy-efficient action potentials in GABAergic interneuron axons. Neuron 98(1):156-165.e6. https://doi.org/10.1016/j.neuron.2018.02.024
Hullfish J, Abenes I, Kovacs S, Sunaert S, De Ridder D, Vanneste S (2018) Functional brain changes in auditory phantom perception evoked by different stimulus frequencies. Neurosci Lett 683:160–167. https://doi.org/10.1016/j.neulet.2018.07.043
Hullfish J, Sedley W, Vanneste S (2019) Prediction and perception: insights for (and from) tinnitus. Neurosci Biobehav Rev 102:1–12. https://doi.org/10.1016/j.neubiorev.2019.04.008
Irvine DRF (2018) Plasticity in the auditory system. Hear Res 362:61–73. https://doi.org/10.1016/j.heares.2017.10.011
Jastreboff PJ, Gray WC, Gold SL (1996) Neurophysiological approach to tinnitus patients. Am J Otol 17(2):236–240
Jeanneteau F, Borie A, Chao MV, Garabedian MJ (2019) Bridging the gap between brain-derived neurotrophic factor and glucocorticoid effects on brain networks. Neuroendocrinology 109(3):277–284. https://doi.org/10.1159/000496392
Kandeepan S, Maudoux A, Ribeiro de Paula D, Zheng JY, Cabay JE, Gómez F, Chronik BA, Ridder D, Vanneste S, Soddu A (2019) Tinnitus distress: a paradoxical attention to the sound? J Neurol 266(9):2197–2207. https://doi.org/10.1007/s00415-019-09390-1
Kann L, McManus T, Harris WA, Shanklin SL, Flint KH, Hawkins J, Queen B, Lowry R, Olsen EOM, Chyen D, Whittle L, Thornton J, Lim C, Yamakawa Y, Brener N, Zaza S (2016) Youth risk behavior surveillance—United States, 2015. MMWR Surveill Summ 65(6):1–180. https://doi.org/10.15585/mmwr.ss6506a1
Khan RA, Husain FT (2020) Tinnitus and cognition: can load theory help us refine our understanding? Laryngosc Invest Otolaryngol 5(6):1197–1204. https://doi.org/10.1002/lio2.501
Kleine Punte A, De Ridder D, Van De Heyning P (2013) On the necessity of full length electrical cochlear stimulation to suppress severe tinnitus in single-sided deafness. Hear Res 295:24–29. https://doi.org/10.1016/j.heares.2012.08.003
Knipper M, van Dijk P, Schulze H, Mazurek B, Krauss P, Scheper V, Warnecke A, Schlee W, Schwabe K, Singer W, Braun C, Delano PH, Fallgatter AJ, Ehlis AC, Searchfield GD, Munk MHJ, Baguley DM, Rüttiger L (2020) The neural bases of tinnitus: lessons from deafness and cochlear implants. J Neurosci 40(38):7190–7202. https://doi.org/10.1523/JNEUROSCI.1314-19.2020
Knopke S, Szczepek AJ, Häussler SM, Gräbel S, Olze H (2017) Cochlear implantation of bilaterally deafened patients with tinnitus induces sustained decrease of tinnitus-related distress. Front Neurol. https://doi.org/10.3389/fneur.2017.00158
Koops EA, van Dijk P (2021) Hyperacusis in tinnitus patients relates to enlarged subcortical and cortical responses to sound except at the tinnitus frequency. Hear Res. https://doi.org/10.1016/j.heares.2020.108158
Koops EA, Renken RJ, Lanting CP, van Dijk P (2020) Cortical tonotopic map changes in humans are larger in hearing loss than in additional tinnitus. J Neurosci 40(16):3178–3185. https://doi.org/10.1523/JNEUROSCI.2083-19.2020
Koops EA, Haykal S, van Dijk P (2021) Macrostructural changes of the acoustic radiation in humans with hearing loss and tinnitus revealed with fixel-based analysis. J Neurosci 41(18):3958–3965. https://doi.org/10.1523/jneurosci.2996-20.2021
Kozlovskiy SA, Pyasik MM, Korotkova AV, Vartanov AV, Glozman JM, Kiselnikov AA (2014) Activation of left lingual gyrus related to working memory for schematic faces. Int J Psychophysiol 94(2):241. https://doi.org/10.1016/j.ijpsycho.2014.08.928
Kraus KS, Canlon B (2012) Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear Res 288(1–2):34–46. https://doi.org/10.1016/j.heares.2012.02.009
Krauss P, Tziridis K, Metzner C, Schilling A, Hoppe U, Schulze H (2016) Stochastic resonance controlled upregulation of internal noise after hearing loss as a putative cause of tinnitus-related neuronal hyperactivity. Front Neurosci. https://doi.org/10.3389/fnins.2016.00597
Krauss P, Metzner C, Schilling A, Schütz C, Tziridis K, Fabry B, Schulze H (2017) Adaptive stochastic resonance for unknown and variable input signals. Sci Rep. https://doi.org/10.1038/s41598-017-02644-w
Krauss P, Metzner C, Schilling A, Tziridis K, Traxdorf M, Wollbrink A, Rampp S, Pantev C, Schulze H (2018a) A statistical method for analyzing and comparing spatiotemporal cortical activation patterns. Sci Rep. https://doi.org/10.1038/s41598-018-23765-w
Krauss P, Tziridis K, Schilling A, Schulze H (2018b) Cross-modal stochastic resonance as a universal principle to enhance sensory processing. Front Neurosci 12(AUG):578. https://doi.org/10.3389/fnins.2018.00578
Land R, Baumhoff P, Tillein J, Lomber SG, Hubka P, Kral A (2016) Cross-modal plasticity in higher-order auditory cortex of congenitally deaf cats does not limit auditory responsiveness to cochlear implants. J Neurosci 36(23):6175–6185. https://doi.org/10.1523/JNEUROSCI.0046-16.2016
Lanting CP, De Kleine E, Langers DRM, Van Dijk P (2014) Unilateral tinnitus: changes in connectivity and response lateralization measured with fMRI. PLoS One. https://doi.org/10.1371/journal.pone.0110704
Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP (2011) Dysregulation of limbic and auditory networks in tinnitus. Neuron 69(1):33–43. https://doi.org/10.1016/j.neuron.2010.12.002
Lee SY, Nam DW, Koo JW, De Ridder D, Vanneste S, Song JJ (2017) No auditory experience, no tinnitus: lessons from subjects with congenital- and acquired single-sided deafness. Hear Res 354:9–15. https://doi.org/10.1016/j.heares.2017.08.002
Lee SY, Choi BY, Koo JW, De Ridder D, Song JJ (2020) Cortical oscillatory signatures reveal the prerequisites for tinnitus perception: a comparison of subjects with sudden sensorineural hearing loss with and without tinnitus. Front Neurosci. https://doi.org/10.3389/fnins.2020.596647
Li C, Kuhlmey M, Kim AH (2019) Electroacoustic stimulation. Otolaryngol Clin North Am 52(2):311–322. https://doi.org/10.1016/j.otc.2018.11.008
Liberman MC (1978) Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am 63(2):442–455. https://doi.org/10.1121/1.381736
Liberman MC, Kujawa SG (2017) Cochlear synaptopathy in acquired sensorineural hearing loss: manifestations and mechanisms. Hear Res 349:138–147. https://doi.org/10.1016/j.heares.2017.01.003
Lopez-Escamez JA, Amanat S (2020) Heritability and genetics contribution to tinnitus. Otolaryngol Clin North Am 53(4):501–513. https://doi.org/10.1016/j.otc.2020.03.003
Luan Y, Wang C, Jiao Y, Tang T, Zhang J, Teng GJ (2019) Prefrontal-temporal pathway mediates the cross-modal and cognitive reorganization in sensorineural hearing loss with or without tinnitus: a Multimodal MRI Study. Front Neurosci. https://doi.org/10.3389/fnins.2019.00222
Majhi SK, Khandelwal K, Shareef M (2019) Auditory brainstem response in patients of tinnitus with sensorineural hearing loss. Indian J Otolaryngol Head Neck Surg 71(Suppl 2):1495–1499. https://doi.org/10.1007/s12070-018-1568-0
Mamashli F, Khan S, Bharadwaj H, Michmizos K, Ganesan S, Garel KLA, Ali Hashmi J, Herbert MR, Hämäläinen M, Kenet T (2017) Auditory processing in noise is associated with complex patterns of disrupted functional connectivity in autism spectrum disorder. Autism Res 10(4):631–647. https://doi.org/10.1002/aur.1714
Marchetta P, Savitska D, Kübler A, Asola G, Manthey M, Möhrle D, Schimmang T, Rüttiger L, Knipper M, Singer W (2020) Age-dependent auditory processing deficits after cochlear synaptopathy depend on auditory nerve latency and the ability of the brain to recruit ltp/bdnf. Brain Sci 10(10):1–26. https://doi.org/10.3390/brainsci10100710
Marks E, Smith P, McKenna L (2019) Living with tinnitus and the health care journey: an interpretative phenomenological analysis. Br J Health Psychol 24(2):250–264. https://doi.org/10.1111/bjhp.12351
Martel DT, Shore SE (2020) Ventral cochlear nucleus bushy cells encode hyperacusis in guinea pigs. Sci Rep. https://doi.org/10.1038/S41598-020-77754-Z
Matt L, Eckert P, Panford-Walsh R, Geisler HS, Bausch AE, Manthey M, Müller NIC, Harasztosi C, Rohbock K, Ruth P, Friauf E, Ott T, Zimmermann U, Rüttiger L, Schimmang T, Knipper M, Singer W (2018) Visualizing BDNF transcript usage during sound-induced memory linked plasticity. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2018.00260
Mazurek B, Haupt H, Olze H, Szczepek AJ (2012) Stress and tinnitus-from bedside to bench and back. Front Syst Neurosci. https://doi.org/10.3389/fnsys.2012.00047
Mazurek B, Boecking B, Brueggemann P (2019) Association between stress and tinnitus-new aspects. Otol Neurotol 40(4):e467–e473. https://doi.org/10.1097/MAO.0000000000002180
Mertens G, Van Rompaey V, Van de Heyning P (2018) Electric-acoustic stimulation suppresses tinnitus in a subject with high-frequency single-sided deafness. Cochlear Implants Int 19(5):292–296. https://doi.org/10.1080/14670100.2018.1473940
Michiels S, Naessens S, Van de Heyning P, Braem M, Visscher CM, Gilles A, De Hertogh W (2016) The effect of physical therapy treatment in patients with subjective tinnitus: a systematic review. Front Neurosci. https://doi.org/10.3389/fnins.2016.00545
Milloy V, Fournier P, Benoit D, Noreña A, Koravand A (2017) Auditory brainstem responses in tinnitus: a review of who, how, and what? Front Aging Neurosci. https://doi.org/10.3389/fnagi.2017.00237
Mohamad N, Hoare DJ, Hall DA (2016) The consequences of tinnitus and tinnitus severity on cognition: a review of the behavioural evidence. Hear Res 332:199–209. https://doi.org/10.1016/j.heares.2015.10.001
Mohebbi M, Daneshi A, Asadpour A, Mohsen S, Farhadi M, Mahmoudian S (2019) The potential role of auditory prediction error in decompensated tinnitus: an auditory mismatch negativity study. Brain Behav 9(4):1–14. https://doi.org/10.1002/brb3.1242
Möhrle D, Hofmeier B, Amend M, Wolpert S, Ni K, Bing D, Klose U, Pichler B, Knipper M, Rüttiger L (2019) Enhanced central neural gain compensates acoustic trauma-induced cochlear impairment, but unlikely correlates with tinnitus and hyperacusis. Neuroscience 407:146–169. https://doi.org/10.1016/j.neuroscience.2018.12.038
Mohsen S, Mahmoudian S, Talbian S, Pourbakht A (2019) Research paper: correlation analysis of the tinnitus handicap inventory and distress network in chronic tinnitus: an EEG Study. Basic Clin Neurosci 10(5):499–514. https://doi.org/10.32598/bcn.9.10.215
Nagaraj MK, Bhaskar A, Prabhu P (2020) Assessment of auditory working memory in normal hearing adults with tinnitus. Eur Arch Otorhinolaryngol 277(1):47–54. https://doi.org/10.1007/s00405-019-05658-4
Neff PKA, Schoisswohl S, Simoes J, Staudinger S, Langguth B, Schecklmann M, Schlee W (2021) Prolonged tinnitus suppression after short-term acoustic stimulation. Prog Brain Res. https://doi.org/10.1016/bs.pbr.2021.02.004
Niemann U, Brueggemann P, Boecking B, Mebus W, Rose M, Spiliopoulou M, Mazurek B (2020) Phenotyping chronic tinnitus patients using self-report questionnaire data: cluster analysis and visual comparison. Sci Rep. https://doi.org/10.1038/s41598-020-73402-8
Norena A, Lacher-Fougère S, Fraysse M-J, Bizaguet E, Grevin P, Thai-Van H, Moati L, Le Pajolec C, Fournier P, Ohresser M (2021) A contribution to the debate on tinnitus definition. Prog Brain Res. https://doi.org/10.1016/bs.pbr.2021.01.029
Noreña AJ, Farley BJ (2013) Tinnitus-related neural activity: theories of generation, propagation, and centralization. Hear Res 295:161–171. https://doi.org/10.1016/j.heares.2012.09.010
Ortmann M, Müller N, Schlee W, Weisz N (2011) Rapid increases of gamma power in the auditory cortex following noise trauma in humans. Eur J Neurosci 33(3):568–575. https://doi.org/10.1111/j.1460-9568.2010.07542.x
Oxenham AJ (2018) How we hear: the perception and neural coding of sound. Annu Rev Psychol 69:27–50. https://doi.org/10.1146/annurev-psych-122216-011635
Park E, Kim H, Choi IH, Han HM, Han K, Jung HH, Im GJ (2020) Psychiatric distress as a common risk factor for tinnitus and joint pain: a national population-based survey. Clin Exp Otorhinolaryngol 13(3):234–240. https://doi.org/10.21053/ceo.2019.00563
Pattyn T, Van Den Eede F, Vanneste S, Cassiers L, Veltman DJ, Van De Heyning P, Sabbe BCG (2016) Tinnitus and anxiety disorders: a review. Hear Res 333:255–265. https://doi.org/10.1016/j.heares.2015.08.014
Peters TTA, Van Den Berge MJC, Free RH, Van Der Vliet AM, Knoppel H, Van Dijk P, Hofman R (2020) The relation between tinnitus and a neurovascular conflict of the cochleovestibular nerve on magnetic resonance imaging. Otol Neurotol 41(1):E124–E131. https://doi.org/10.1097/MAO.0000000000002432
Pillsbury HC, DIllon MT, Buchman CA, Staecker H, Prentiss SM, Ruckenstein MJ, Bigelow DC, Telischi FF, Di Martinez M, Runge CL, Friedland DR, Blevins NH, Larky JB, Alexiades G, Kaylie DM, Roland PS, Miyamoto RT, Backous DD, Warren FM et al (2018) Multicenter US Clinical Trial with an electric-acoustic stimulation (EAS) system in adults: final outcomes. Otol Neurotol 39(3):299–305. https://doi.org/10.1097/MAO.0000000000001691
Ralli M, Salvi RJ, Greco A, Turchetta R, De Virgilio A, Altissimi G, Attanasio G, Cianfrone G, De Vincentiis M (2017) Characteristics of somatic tinnitus patients with and without hyperacusis. PLoS One. https://doi.org/10.1371/journal.pone.0188255
Ramakers GGJ, Van Zon A, Stegeman I, Grolman W (2015) The effect of cochlear implantation on tinnitus in patients with bilateral hearing loss: a systematic review. Laryngoscope 125(11):2584–2592. https://doi.org/10.1002/lary.25370
Rauschecker JP (2010) An expanded role for the dorsal auditory pathway in sensorimotor control and integration. Hear Res. https://doi.org/10.1016/j.heares.2010.09.001
Rauschecker JP, Fritz JB, Garraghty PE, Freedman DJ (2014) Is there a tape recorder in your head? How the brain stores and retrieves musical melodies. Front Syst Neurosci. https://doi.org/10.3389/fnsys.2014.00149
Refat F, Wertz J, Hinrichs P, Klose U, Samy H, Abdelkader RM, Saemisch J, Hofmeier B, Singer W, Rüttiger L, Knipper M, Wolpert S (2021) Co-occurrence of hyperacusis accelerates with tinnitus burden over time and requires medical care. Front Neurol. https://doi.org/10.3389/fneur.2021.627522
Riffle TL, Martel DT, Jones GR, Shore SE (2020) Bimodal auditory electrical stimulation for the treatment of tinnitus: preclinical and clinical studies. Curr Top Behav Neurosci. https://doi.org/10.1007/7854_2020_180
Rossignol E, Kruglikov I, Van Den Maagdenberg AMJM, Rudy B, Fishell G (2013) CaV2.1 ablation in cortical interneurons selectively impairs fast-spiking basket cells and causes generalized seizures. Ann Neurol 74(2):209–222. https://doi.org/10.1002/ana.23913
Ruan Q, Yu Z, Zhang W, Ruan J, Liu C, Zhang R (2018) Cholinergic hypofunction in presbycusis-related tinnitus with cognitive function impairment: emerging hypotheses. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2018.00098
Rüttiger L, Singer W, Panford-Walsh R, Matsumoto M, Lee SC, Zuccotti A, Zimmermann U, Jaumann M, Rohbock K, Xiong H, Knipper M (2013) The reduced cochlear output and the failure to adapt the central auditory response causes tinnitus in noise exposed rats. PLoS ONE. https://doi.org/10.1371/journal.pone.0057247
Schaette R, McAlpine D (2011) Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci 31(38):13452–13457. https://doi.org/10.1523/JNEUROSCI.2156-11.2011
Schecklmann M, Landgrebe M, Kleinjung T, Frank E, Sand PG, Rupprecht R, Eichhammer P, Hajak G, Langguth B (2014) Changes in motor cortex excitability associated with temporal repetitive transcranial magnetic stimulation in tinnitus: hints for cross-modal plasticity? BMC Neurosci. https://doi.org/10.1186/1471-2202-15-71
Schilling A, Tziridis K, Schulze H, Krauss P (2021) The stochastic resonance model of auditory perception: a unified explanation of tinnitus development, Zwicker tone illusion, and residual inhibition. Prog Brain Res. https://doi.org/10.1016/bs.pbr.2021.01.025
Schmidt SA, Akrofi K, Carpenter-Thompson JR, Husain FT (2013) Default mode, dorsal attention and auditory resting state networks exhibit differential functional connectivity in tinnitus and hearing loss. PLoS ONE. https://doi.org/10.1371/journal.pone.0076488
Schmidt SA, Carpenter-Thompson J, Husain FT (2017) Connectivity of precuneus to the default mode and dorsal attention networks: a possible invariant marker of long-term tinnitus. NeuroImage Clin 16:196–204. https://doi.org/10.1016/j.nicl.2017.07.015
Searchfield GD (2014) Tinnitus what and where: an ecological framework. Front Neurol. https://doi.org/10.3389/fneur.2014.00271
Searchfield GD, Kaur M, Martin WH (2010) Hearing aids as an adjunct to counseling: tinnitus patients who choose amplification do better than those that don’t. Int J Audiol 49(8):574–579. https://doi.org/10.3109/14992021003777267
Searchfield GD, Fok C, Donaldson T, Durai M, Kleinstäuber M, Linford T, Maslin M (2020) An evaluation of a continuing education workshop for audiologists on the assessment and management of tinnitus. J Contin Educ Health Prof 40(2):125–130. https://doi.org/10.1097/CEH.0000000000000285
Sedley W, Gander PE, Kumar S, Kovach CK, Oya H, Kawasaki H, Howard MA, Griffiths TD (2016) Neural signatures of perceptual inference. Elife. https://doi.org/10.7554/eLife.11476
Shekhawat GS, Searchfield GD, Stinear CM (2013) Role of hearing aids in tinnitus intervention: a scoping review. J Am Acad Audiol 24(8):747–762. https://doi.org/10.3766/jaaa.24.8.11
Shore SE, Roberts LE, Langguth B (2016) Maladaptive plasticity in tinnitus-triggers, mechanisms and treatment. Nat Rev Neurol 12(3):150–160. https://doi.org/10.1038/nrneurol.2016.12
Simoens VL, Hébert S (2012) Cortisol suppression and hearing thresholds in tinnitus after low-dose dexamethasone challenge. BMC Ear Nose Throat Disord. https://doi.org/10.1186/1472-6815-12-4
Singer W, Zuccotti A, Jaumann M, Lee SC, Panford-Walsh R, Xiong H, Zimmermann U, Franz C, Geisler HS, Köpschall I, Rohbock K, Varakina K, Verpoorten S, Reinbothe T, Schimmang T, Rüttiger L, Knipper M (2013) Noise-induced inner hair cell ribbon loss disturbs central arc mobilization: a novel molecular paradigm for understanding tinnitus. Mol Neurobiol 47(1):261–279. https://doi.org/10.1007/s12035-012-8372-8
Spankovich C, Le Prell CG (2019) The role of diet in vulnerability to noise-induced cochlear injury and hearing loss. J Acoust Soc Am 146(5):4033–4043. https://doi.org/10.1121/1.5132707
Szczepek AJ, Frejo L, Vona B, Trpchevska N, Cederroth CR, Caria H, Lopez-Escamez JA (2019) Recommendations on collecting and storing samples for genetic studies in hearing and tinnitus research. Ear Hear 40(2):219–226. https://doi.org/10.1097/AUD.0000000000000614
Tran AN, Koo JY (2014) Risk of systemic toxicity with topical lidocaine/prilocaine: a review—PubMed. J Drugs Dermatol 13(9):1118–1122
Trevis KJ, McLachlan NM, Wilson SJ (2016) Psychological mediators of chronic tinnitus: the critical role of depression. J Affect Disord 204:234–240. https://doi.org/10.1016/j.jad.2016.06.055
Tyler RS, Rubinstein J, Pan T, Chang SA, Gogel SA, Gehringer A, Coelho C (2008) Electrical stimulation of the cochlea to reduce tinnitus. Semin Hear 29(4):326–332. https://doi.org/10.1055/s-0028-1095892
Van Den Berge MJC, Van Dijk MJMC, Metzemaekers JDM, Maat B, Free RH, Van Dijk P (2019) An auditory brainstem implant for treatment of unilateral tinnitus: protocol for an interventional pilot study. BMJ Open. https://doi.org/10.1136/bmjopen-2018-026185
van Gendt MJ, Boyen K, de Kleine E, Langers DRM, van Dijk P (2012) The relation between perception and brain activity in gaze-evoked tinnitus. J Neurosci 32(49):17528–17539. https://doi.org/10.1523/JNEUROSCI.2791-12.2012
van Munster JJCM, van der Valk WH, Stegeman I, Lieftink AF, Smit AL (2020) The relationship of tinnitus distress with personality traits: a systematic review. Front Neurol 11:1–40. https://doi.org/10.3389/fneur.2020.00225
Vanneste S, Alsalman O, De Ridder D (2018a) COMT and the neurogenetic architecture of hearing loss induced tinnitus. Hear Res 365:1–15. https://doi.org/10.1016/j.heares.2018.05.020
Vanneste S, Song JJ, De Ridder D (2018b) Thalamocortical dysrhythmia detected by machine learning. Nat Commun. https://doi.org/10.1038/s41467-018-02820-0
Vanneste S, Alsalman O, De Ridder D (2019) Top-down and bottom-up regulated auditory phantom perception. J Neurosci 39(2):364–378. https://doi.org/10.1523/JNEUROSCI.0966-18.2018
Vanneste S, Mohan A, De Ridder D, To WT (2021) The BDNF Val66Met polymorphism regulates vulnerability to chronic stress and phantom perception. Prog Brain Res 260:301–326. https://doi.org/10.1016/bs.pbr.2020.08.005
Vielsmeier V, Santiago Stiel R, Kwok P, Langguth B, Schecklmann M (2020) From acute to chronic tinnitus: pilot data on predictors and progression. Front Neurol. https://doi.org/10.3389/fneur.2020.00997
Vielsmeier V, Schlee W, Langguth B, Kreuzer PM, Hintschich C, Strohmeyer L, Simoes J, Biesinger E (2021) Lidocaine injections to the otic ganglion for the treatment of tinnitus—a pilot study. Prog Brain Res 260:355–366. https://doi.org/10.1016/bs.pbr.2020.08.006
Wallhäusser-Franke E, Delb W, Balkenhol T, Hiller W, Hörmann K (2014) Tinnitus-related distress and the personality characteristic resilience. Neural Plast. https://doi.org/10.1155/2014/370307
Weisz N, Dohrmann K, Elbert T (2007) The relevance of spontaneous activity for the coding of the tinnitus sensation. Prog Brain Res 166:61–70. https://doi.org/10.1016/S0079-6123(07)66006-3
White O, Babic J, Trenado C, Johannsen L, Goswami N (2019) The promise of stochastic resonance in falls prevention. Front Physiol. https://doi.org/10.3389/fphys.2018.01865
Wilting J, Dehning J, Neto JP, Rudelt L, Wibral M, Zierenberg J, Priesemann V (2018) Dynamic adaptive computation: tuning network states to task requirements. arXiv. https://doi.org/10.3389/fnsys.2018.00055
Wu PZ, Liberman LD, Bennett K, de Gruttola V, O’Malley JT, Liberman MC (2019) Primary neural degeneration in the human cochlea: evidence for hidden hearing loss in the aging ear. Neuroscience 407:8–20. https://doi.org/10.1016/j.neuroscience.2018.07.053
Yoo H. Bin, Mohan A, De Ridder D, Vanneste S (2021) Paradoxical relationship between distress and functional network topology in phantom sound perception. Prog Brain Res 260:367–395. https://doi.org/10.1016/bs.pbr.2020.08.007
Zeng FG (2020) Tinnitus and hyperacusis: central noise, gain and variance. Curr Opin Physiol 18:123–129. https://doi.org/10.1016/j.cophys.2020.10.009
Acknowledgements
MK received funding from Deutsche Forschungsgemeinschaft KN316/13-1 ; ERA-NET Neuron, Neuron-023 CoSy Speech (BMBF 01EW2102).
BM, PvD and HS received funding from the European Research Council (ERC) under the European Union‘s Horizon 2020 Research and Innovation Programme (Grant Agreement No 764604, TIN-ACT). PvD was supported by the Heinsius Houbolt Foundation. Language services were provided by stels-ol.de. We thank the contributors of the symposium held at the 44th Virtual MidWinter Meeting, February 20–24, 2021, particularly those who addressed the concrete questions discussed in the symposium which formed the topics of the manuscript: Eberhard Biesinger, HNO-Praxis Lindenberg; Don Caspary, Southern Illinois University School of Medicine; Frederic De Martino, University of Maastricht; Jos Eggermont, University of Calgary; Sylvie Hébert, University of Montreal; Fatima Husain, University of Illinois; Pawel Jastreboff, Emroy University School of Medicine; Michael Kilgard, University of Texas at Dallas; Elouise Koops, University of Groningen; Berthold Langguth, University of Regensburg; Po-Hung Li, National Yang-Ming Chiao-Tung University; Michelle Moerel, University of Maastricht; Arnaud Noreña, Aix-Marseille University; Bryan Pollard, Hyperacusis Research Limted, Inc; Marlboro, MA, USA; Josef Rauschecker, Technical University of Munich; Richard Salvi, University of Buffalo; Will Sedley, Newcastle University; Barbara Shin-Cunningham, Carnegie Mellon University; Robert J. Stokroos, University Medical Center Utrecht; Josef Syka, Czech Academy of Sciences; Sarah Verhulst, Ghent University; Jinsheng Zhang, Wayne State University.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All authors have shared correspondence.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Knipper, M., Mazurek, B., van Dijk, P. et al. Too Blind to See the Elephant? Why Neuroscientists Ought to Be Interested in Tinnitus. JARO 22, 609–621 (2021). https://doi.org/10.1007/s10162-021-00815-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-021-00815-1