Abstract
Background
To suppress the incidence of end-stage kidney disease, we need to identify chronic kidney disease (CKD) patients with a high risk of rapid decline in the estimated glomerular filtration rate (eGFR). However, the current status of eGFR slope and its associated factors in the Japanese population have not been fully elucidated.
Methods
Among examinees aged 40–70 years in the 2014 Specific Health Checkup conducted by the National Health Insurance in Kobe, Japan (n = 61,985), we prospectively observed 7291 examinees with CKD stage G3 from 2014 to 2018.
Results
Until 2018, 4221 examinees continued to undergo annual SHCs for a total of five checkups per subject and had available records of all necessary data. The median eGFR change was −0.22 ml/min/1.73 m2/year. Only 9.2% of those subjects showed rapid eGFR decline (faster than −2.0 ml/min/1.73 m2/year). Logistic regression analysis identified diabetes, smoking habits, high urinary protein levels, older age, high systolic blood pressure, and low serum low-density lipoprotein cholesterol levels as independent predictors for rapid eGFR decline. Hemoglobin A1c levels did not contribute to the eGFR slope in CKD stage-G3 subjects with diabetes and proteinuria.
Conclusion
Most Japanese CKD stage-G3 subjects had a very slow decline in eGFR. A small proportion of CKD individuals who have a predictive factor of rapid eGFR decline should receive considerable attention from a nephrologist.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) affects 14.8 million individuals in Japan [1]. Although the number of incident dialysis patients has decreased, the prevalent number of dialysis patients continues to increase by approximately 2000 every year in the country; thus, measures to prevent CKD progression are urgently needed. However, it is not feasible for a few nephrologists to manage such a large number of CKD patients. In fact, there are many patients with a very slow decline in the estimated glomerular filtration rate (eGFR), even in CKD stage G3 or beyond. To suppress the incidence of end-stage kidney disease (ESKD) requiring renal replacement therapies (RRTs) with limited medical resources, it is necessary to explore the predictors of rapidly progressive CKD to determine what types of CKD patients should receive the most attention.
In Japan, all citizens are covered by health insurance. Since 2008, medical insurers have been obliged to provide the Specific Health Checkup (SHC) focusing on metabolic syndrome for all their subscribers aged 40–74 years. This program plays an important role in that it not only provides healthcare guidance based on individual results but also improves prognoses in patients with lifestyle-related diseases by analyzing a large population dataset.
As an indicator of kidney disease progression, eGFR slope is useful. The slope not only serves as a surrogate marker for the development of ESKD, but also is widely used in daily clinical practice such as predicting kidney prognoses, planning treatment strategies, evaluating treatment effects, and patient education. In this study, we revealed the eGFR slope in each clinical status in CKD stage-G3 subjects of Japanese by analyzing the SHC dataset.
Materials and methods
Study cohort
Of the 655,796 inhabitants aged 40 to 70 in Kobe City, Japan in 2014, approximately 196,000 had been covered by National Health Insurance (NHI), and 61,985 individuals underwent the 2014 NHI-conducted SHC in Kobe. The checkup rate was 31.6%. Of those 61,985 examinees, we prospectively observed the annual SHC results of 7291 examinees with CKD stage G3, from 2014 to 2018 (Fig. 1).
Clinical indicators
The data obtained from SHCs, such as systolic blood pressure (SBP), serum low-density lipoprotein cholesterol (sLDL-C), serum uric acid (sUA), hemoglobin A1c (HbA1c) levels, body mass index (BMI), and urinary protein measured by the dip-stick method, were used for each analysis as clinical indicators. Diabetes was defined as HbA1c ≥ 6.5% or use of antidiabetic drugs. A smoking habit was defined as smoking within six months before the start of the observation.
Evaluation of kidney function
The serum creatinine (sCr) value was measured by the enzymatic method and recorded to two decimal places in the SHC. We calculated an eGFR value for each examinee using the Japanese equation for eGFR from sCr as follows [2]:
We calculated the mean annual change in eGFR (ΔeGFR; ml/min/1.73 m2/year) based on the slope of the regression line of eGFR values from five consecutive annual SHCs.
For the estimation of kidney prognosis, we calculated the predicted eGFR at the end of life (predicted end eGFR) for each subject as follows:
The mean life expectancy of Japanese individuals was 81.3 years for males and 87.3 years for females in 2018. Since the average eGFR value at the time of dialysis initiation in Japan was 5.43 ml/min/1.73 m2 [3], we defined the subjects whose predicted end eGFR values would be <5 ml/min/1.73 m2 as “RRT high-risk subjects” in this study.
In our preliminary analysis in this study, RRT high-risk subjects were divided into groups of 1 ml/min/1.73 m2/year of ΔeGFR for screening. {sensitivity-(1-specificity)} was at its maximum (sensitivity 92.9%, specificity 94.6%, odds ratio 229.1, 95% CI 128.3–409.4) when −2 ml/min/1.73 m2/year of ΔeGFR was set as the cutoff value. Therefore, we defined a subject whose ΔeGFR was at that level or faster as a “rapid GFR decliner (RGD)” in this study.
Statistical analyses
To compare the ΔeGFR distribution according to clinical status, the Mann–Whitney U test for comparisons between two groups and the Kruskal–Wallis test among three or more groups were performed. In diabetes subjects with CKD stage G3 and proteinuria, to explore independent factors that contributed to ΔeGFR, linear regression analysis was performed. In addition, we identified RRT high-risk subjects and RGDs among all CKD stage-G3 subjects. To explore independent predictors of RGD among the clinical indicators measured in SHCs, logistic regression analysis was performed. All statistical analyses were performed using IBM SPSS Statistics 26 (IBM Corp., USA). In each analysis, a P value <0.05 indicated statistical significance.
Results
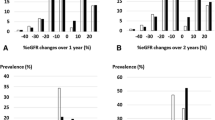
Of 7291 examinees with CKD stage G3 in the 2014 SHC in Kobe, 4221 examinees had continued to undergo annual SHCs until 2018, for a total of five checkups per examinee, and had available records of all necessary data (Fig. 1). The baseline characteristics of the 4221 subjects with stage G3 CKD are described in Table 1. The distribution of ΔeGFR values is shown in Fig. 2. The overall median ΔeGFR was −0.22 ml/min/1.73 m2/year. During the study period, seven subjects progressed to CKD stage G5, but none of them reached an eGFR of <5 ml/min/1.73 m2. In the 4221 subjects, the proportions of RGDs and RRT high-risk subjects were 9.2 and 4.3%, respectively. Figure 3 shows the median ΔeGFR, the proportion of RGDs, and the proportion of RRT high-risk subjects stratified by CKD stages. The distribution of ΔeGFR in each clinical status is shown in Table 2. In males, age, baseline eGFR, baseline SBP, and baseline urinary protein levels were significantly correlated with ΔeGFR. In females, baseline urinary protein levels and a smoking habit were significantly correlated with ΔeGFR. Although no significant difference in the ΔeGFR distribution was found in the overall comparison of subjects with and without diabetes, when limited to subjects with proteinuria, the decline in eGFR was significantly faster in diabetic subjects than in nondiabetic subjects (Table 3). In 69 diabetic subjects with CKD stage G3 and proteinuria, none of the factors, including HbA1c, were associated with ΔeGFR in the linear regression analysis (Table 4).
Median ΔeGFR, rapid GFR decliners, and renal replacement therapy high-risk subjects in each CKD stage. ΔeGFR mean annual change in estimated glomerular filtration rate, GFR glomerular filtration rate, CKD chronic kidney disease, U-P urinary protein, RD rapid glomerular filtration rate decliner, RRT risk renal replacement therapy high-risk subject ΔeGFR are indicated as median values and interquartile ranges
A logistic regression analysis on the proportion of RGDs showed that diabetes (P < 0.001, odds ratio: OR 2.15), smoking habits (P = 0.002, OR 1.72), a higher baseline urinary protein level (P < 0.001, OR 1.58 per step), older age (P = 0.01, OR 1.51 per 10 years), a higher baseline SBP (P = 0.008, OR 1.09 per 10 mmHg), and a lower baseline sLDL-C level (P = 0.01, OR 1.04 per 10 mg/dl) were independent predictors (Table 5).
Discussion
We used ΔeGFR as an indicator of changes in kidney function because it can be utilized easily in a wide range of daily clinical practices as mentioned in the introduction section. The noteworthy point of the present study is that the decline rate in eGFR was remarkably slower than previous reports. Since the early 2000s, various epidemiological studies on CKD have been reported. In Norway, the mean ΔeGFR, determined using a hospital database, for patients with CKD stage G3 was −1.03 ml/min/1.73 m2/year [4]. In elderly individuals with CKD stage G3 in Canada, the mean ΔeGFRs were −3.6 and −2.8 mL/min/1.73 m2/year in males and females with diabetes, respectively, and −1.9 and −1.1 mL/min/1.73 m2/year in males and females without diabetes, respectively [5]. In a previous study in the general Japanese population, using a database of health checkups of the two periods over 10 years, 1988–1993 and 1998–2003, the mean ΔeGFR was as slow as −0.36 ml/min/1.73 m2/year [6]. It is not clear why eGFR declines slower in Japanese CKD patients than in Western CKD patients. However, some differences in background factors of Japanese CKD patients compared to Western CKD patients has been reported, such as low complication rate of arteriosclerotic disease, low BMI, low serum CRP levels [7, 8]. It is speculated that those factors may have affected the differences in eGFR slope between races. In our results, ΔeGFR (median; −0.22 ml/min/1.73 m2/year, mean; −0.19 ml/min/1.73 m2/year) in CKD stage-G3 subjects was even slower than the result of similar study previously conducted in Japan using health checkup data [6]. Since 2002, awareness of CKD has increased, and total CKD management has become widespread. The difference in the results between the previous report and the present study may reflect the advances over the last two decades. In addition, all the subjects in this study underwent the SHC every year, and those who met the criteria for metabolic syndrome had opportunities to receive healthcare guidance through a public health nurse. The very slow decline in eGFR across this population may have resulted from the program.
The Kidney Disease Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guideline for the Evaluation and Management of CKD defined rapid progression as a sustained decline in eGFR of 5 ml/min/1.73 m2/year or more [9]. However, few studies are available regarding the optimal definition of rapid progression [9]. According to an analysis of 529,312 individuals using the Alberta Kidney Disease Network database cited in the 2012 KDIGO guideline, the risk of ESKD increased continuously by approximately twofold for every 1 ml/min/1.73 m2/year decrease in ΔeGFR, and the basis for the cutoff value of 5 ml/min/1.73 m2/year is not clear. When ΔeGFR < -2 ml/min/1.73 m2/year, the risk of ESKD was shown to be 2.71 times (2.08–3.53) compared to a group with stable kidney function [9], but a large number of individuals with eGFR ≥ 60 ml/min/1.73 m2 had been included in the study subjects. The risk is therefore presumed to be even higher in individuals with CKD stage G3. In the present study, we determined the cutoff value for RGD as ΔeGFR < −2 ml/min/1.73 m2/year, as we mentioned in the materials and methods section.
In addition, we also attempted to analyze according to the KDIGO’s definition of rapid progression, but the analysis could not be performed because only 28 of the 4221 subjects (0.66%) had ΔeGFR < −5 ml/min/1.73 m2/year. While in a cohort study in the UK, it has been reported that 24% of CKD cases aged 60 and over had a ΔeGFR < −5 ml/min/1.73 m2/year [10], in the present study, there were extremely few subjects with ΔeGFR < −5 ml/min/1.73 m2/year. Considering that the total number of ESKD patients is nevertheless increasing in Japan, for CKD stage G3 of Japanese, it may be insufficient to focus only on individuals with ΔeGFR < −5 ml/min/1.73 m2/year.
In unadjusted analyses, a baseline urinary protein level was significantly correlated with ΔeGFR in both sexes. Then, among nondiabetic subjects, eGFR declined twice as fast in subjects with proteinuria compared with those without proteinuria, and among diabetic subjects, those with proteinuria had a fivefold faster decline in eGFR than those without proteinuria. In the logistic regression analysis, diabetes and urinary protein levels were significantly associated with the proportion of RGDs irrespective of baseline eGFR, and we consider those factors to be strong predictors of rapid GFR decline, as has been previously reported [5,6,7]. In diabetic subjects with CKD stage G3 and proteinuria, HbA1c levels did not contribute to ΔeGFR. To date, it has been reported that HbA1c levels are associated with the development of albuminuria, and that glycemic control suppresses progression to the microalbuminuria or overt proteinuria phase and reverses from the microalbuminuria phase to the normal-urine phase [11,12,13,14]. However, there is not enough evidence that the eGFR slope is improved by strict blood glucose control after the overt proteinuria phase or once kidney function begins to decrease. Our results are consistent with those previous findings.
A large-scale observation showed that smoking was a risk factor for the progression of CKD [15]. Also, in our study population, having a smoking habit was identified as an independent predictor of RGD. However, in this study, there were no available data on the number of cigarettes smoked, duration of smoking, and smoking history. More detailed data are needed to determine the association between smoking habits and ΔeGFR and the effect of smoking cessation on improving the eGFR slope.
In unadjusted analyses, a higher baseline SBP was significantly correlated with a faster decline in eGFR in males. While, the correlation between those two variables did not show a statistically significant difference in females. The results may have been influenced by the fact that a large proportion of hypertensive subjects in our study population had already taken antihypertensive drugs, and only 2.9 and 1.5% of female subjects had a baseline SBP and a mean SBP of 160 mmHg or higher, respectively. In the logistic regression analysis, a high baseline SBP was significantly associated with the risk of RGD. Thus, there is no doubt that high BP is an important factor that exacerbates CKD, as previously reported [7, 16, 17].
A lower baseline sLDL-C level was associated with a higher risk of RGD in our study population. The result was inconsistent with the findings of a meta-analysis that showed that high sLDL-C was associated with a high risk of CKD [18]. However, in our study, the statistical significance disappeared in an analysis excluding the subjects with baseline sLDL-C levels below 70 mg/dl (n = 96, median ΔeGFR; −0.58 ml/min/1.73 m2/year). Given the above, we speculated not that a higher sLDL-C level was preferable but rather that underlying factors of an extremely low sLDL-C level, such as undernutrition or chronic inflammation, may have contributed to the rapid decline in eGFR. To verify this hypothesis, further investigation, including evaluation of nutritional status, serum albumin, and serum C-reactive protein, is needed. In addition, in our study population, the lower the sLDL-C-level group, the higher the rate of taking a lipid-lowering drug (Pearson’s χ2 test; p < 0.001), suggesting that past histories of high sLDL-C levels may have affected the study results.
The strength of the present study is that the ΔeGFR values are considered high confidence because those were determined using the slopes of the regression lines of the eGFR values of five consecutive times at 1-year intervals over four years, for 4221 subjects. The target area, Kobe City, includes a range of environments from urban to rural. The population per nephrologist of approximately 24,000 was almost the same as the national average (approximately 25,000), and the number of chronic dialysis patients per million people covered by the NHI in Kobe City in 2018 was 2673, which was nearly equal to the national average of 2688. It is presumed that the situation of medical care for CKD in the target area of this study is not remarkably different from the situation in Japan as a whole.
However, the participation rate for the 2014 SHC in Kobe was 31.6%, and thus, the existence of selection bias cannot be denied, which is the biggest limitation in this study. Moreover, it is impossible to follow the examinees who had discontinued SHC participation during the observation period.
There were also no urinary albumin quantification data, and we used a dipstick urine test to evaluate proteinuria. It was reported that urinary protein by a dipstick test of ± or above had a high specificity (95.6%) but low sensitivity (66.2%) and low positive predictive value (17.5%) to detect CKD stage A2 or higher [19]; thus, its inferior accuracy is also a limitation.
The rate of GFR decline may be relatively constant over time in an individual [9, 20]. However, there is no evidence that eGFR slope accurately predicts the time to ESKD. It was reported that the longer the follow-up time, the higher the probability of non-linear trajectory of eGFR decline [9, 21]. The causes of nonlinearity include not only external factors such as intercurrent illness and changes in medication, but also intrinsic to the disease process [21]. Although the present study used data over a relatively long period of time, the accuracy of very long-term predictions on eGFR at the end of life requires further verification.
It is an important issue how many subjects who had transitioned from acute kidney injury (AKI) to CKD among RGDs in the present study. However, in the present study, there was no information on the history of AKI, and only the data at 1-year intervals were available. Thus, it was difficult to determine whether the subjects had developed AKI.
The use of renin-angiotensin system (RAS) inhibitors and sodium glucose co-transporter 2 (SGLT2) inhibitors greatly affects the eGFR slope in CKD patients. However, the SHC dataset used in this study only contained information on the overall use of antihypertensive and antidiabetic drugs, and information on the use of RAS inhibitors and SGLT2 inhibitors was not included. In this study population, it is considered that a substantial number of subjects had been prescribed RAS inhibitors. Although the kidney protective effect of SGLT2 inhibitors in nondiabetic CKD patients had not been revealed until 2020 [22], the use of SGLT2 inhibitors had already been widespread in diabetic patients during the observation period (from 2014 to 2018). Therefore, it is speculated that our results were affected by SGLT2 inhibitors in some subjects with diabetes. The lack of information regarding RAS inhibitors and SGLT2 inhibitors is an important limitation of the present study.
Conclusion
Among subjects with CKD stage G3 in Japan, the decline rate in eGFR (median 0.22 ml/min/1.73 m2/year) was remarkably slower than the result of similar study previously conducted in Japan. Only 9.2% of study subjects had ΔeGFR < −2 ml/min/1.73 m2/year. These results may reflect the advances of countermeasures against CKD in Japan. In nondiabetic subjects, eGFR declined twice as fast in those with proteinuria compared with those without proteinuria, and in diabetic subjects, those with proteinuria had a fivefold faster decline in eGFR than those without proteinuria. In diabetic subjects with CKD stage G3 and proteinuria, HbA1c levels did not contribute to ΔeGFR. A logistic regression analysis revealed that independent predictors for RGD were diabetes, smoking habits, proteinuria, older age, and hypertension, as previously reported. Regarding the significance of a low sLDL-C level for RGD, further investigation is required. To efficiently suppress the number of incident ESKD patients with limited medical resources, we consider it important to preferentially refer those with predictors to a nephrologist.
Data availability
The raw data analyzed during the present study are not publicly available according to the ethical approval and contract with Kobe City. If anyone has questions about the data, please contact the author.
References
Nagai K, Asahi K, Iseki K, Yamagata K. Estimating the prevalence of definitive chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2021;25:885–92.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Nakai S, Masakane I, Shigematsu T, Hamano T, Yamagata K, Watanabe Y, et al. An overview of regular dialysis treatment in Japan (as of 31 December 2007). Ther Apher Dial. 2009;13:457–504.
Eriksen B, Ingebretsen O. The progression of chronic kidney disease: A 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69:375–82.
Hemmelgarn BR, Zhang J, Manns BJ, Tonelli M, Larsen E, Ghali WA, et al. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006;69:2155–61.
Imai E, Horio M, Yamagata K, Iseki K, Hara S, Ura N, et al. Slower decline of glomerular filtration rate in the Japanese general population: a longitudinal 10-year follow-up study. Hypertens Res. 2008;31:433–41.
Inaguma D, Imai E, Takeuchi A, Ohashi Y, Watanabe T, Nitta K, et al. Risk factors for CKD progression in Japanese patients: findings from the Chronic Kidney Disease Japan Cohort (CKD-JAC) study. Clin Exp Nephrol. 2017;21:446–56.
Imaizumi T, Fujii N, Hamano T, Yang W, Taguri M, Kansal M, et al. Excess risk of cardiovascular events in patients in the United States vs. Japan with chronic kidney disease is mediated mainly by left ventricular structure and function. Kidney Int. 2023;103:949–61.
Chapter 2: Definition, identification, and prediction of CKD progression. Kidney Int Suppl. 2013;3:63–72.
Hirst JA, Taal MW, Fraser SD, Mena JMO, O’Callaghan CA, McManus RJ, et al. Change in glomerular filtration rate over time in the Oxford Renal Cohort Study: observational study. Br J Gen Pract. 2022;72:e261–8.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–17.
Katayama S, Moriya T, Tanaka S, Tanaka S, Yajima Y, Sone H, et al. Low transition rate from normo- and low microalbuminuria to proteinuria in Japanese type 2 diabetic individuals: the Japan Diabetes Complications Study (JDCS). Diabetologia. 2011;54:1025–31.
Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339:69–75.
Ricardo AC, Anderson CA, Yang W, Zhang X, Fischer MJ, Dember LM, et al. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2015;65:412–24.
Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation hypertension and diabetes executive committees working group. Am J Kidney Dis. 2000;36:646–61.
Hirayama A, Konta T, Kamei K, Suzuki K, Ichikawa K, Fujimoto S, et al. Blood pressure, proteinuria, and renal function decline: associations in a large community-based population. Am J Hypertens. 2015;28:1150–6.
Emanuelsson F, Nordestgaard BG, Tybjaerg-Hansen A, Benn M. Impact of LDL cholesterol on microvascular versus macrovascular disease: a mendelian randomization study. J Am Coll Cardiol. 2019;74:1465–76.
Usui T, Yoshida Y, Nishi H, Yanagimoto S, Matsuyama Y, Nangaku M. Diagnostic accuracy of urine dipstick for proteinuria category in Japanese workers. Clin Exp Nephrol. 2020;24:151–6.
Baba M, Shimbo T, Horio M, Ando M, Yasuda Y, Komatsu Y, et al. Longitudinal study of the decline in renal function in healthy subjects. PLoS ONE. 2015;10: e0129036.
Li L, Astor BC, Lewis J, Hu B, Appel LJ, Lipkowitz MS, et al. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis. 2012;59:504–12.
Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–46.
Acknowledgements
We would like to express our gratitude to Akinori Masahira, Chief of Research Support Section, Hyogo University, for his support in preparing the office documents.
Funding
No applicable.
Author information
Authors and Affiliations
Contributions
Jun Ito performed all aspects of the present study, including the conception and design of the study, the acquisition of data, the analysis and interpretation of data, and manuscript writing. Masafumi Fukagawa played an important role in interpreting the results, revised the manuscript critically, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors have declared that no conflict of interest exists.
Ethical approval
The present study was approved by the Institutional Review Board of Hyogo University (approval number 13016) in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards, as well as the “Ethical Guidelines for Medical and Health Research Involving Human Subjects” by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare of Japan. The study was carried out based on a contract between Kobe City and Hyogo University. The deidentified data from the SHC were provided to the researchers. Through a message on the website of Kobe City and posters in the SHC venues, all examinees who underwent SHCs by the NHI in Kobe City between 2014 and 2018 were notified of the purpose of this study, the content, the method of data collection, and the opportunity for examinees to exclude their data from the study without any disadvantage.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ito, J., Fukagawa, M. Slope of the estimated glomerular filtration rate and its associated factors among individuals with chronic kidney disease in the general Japanese population. Clin Exp Nephrol 28, 522–530 (2024). https://doi.org/10.1007/s10157-024-02466-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-024-02466-x