Abstract
Background
Atypical hemolytic uremic syndrome (aHUS) is an ultra-rare and life-threatening disease. For decades, plasma therapy was used to manage patients with aHUS. Since eculizumab, a recombinant humanized anti-C5 monoclonal antibody, was approved for treatment of aHUS, it has been used to treat patients with aHUS. Here, we examined the effectiveness of eculizumab and plasma therapy, respectively in the treatment of pediatric patients with aHUS.
Methods
Data were collected from questionnaires sent to 75 institutions known to be treating thrombotic microangiopathy (TMA).
Results
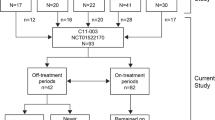
A total of 24 patients were evaluable, in which no recurrence of TMA was reported at last observation. There were four therapy groups: two patients receiving supportive therapy, one receiving plasma therapy alone, 17 switching from plasma therapy to eculizumab (therapy switched), and four receiving eculizumab alone. Among 17 patients of therapy-switched group, only one patient achieved complete remission at the end of plasma therapy, 15 patients achieved complete remission after eculizumab initiation, and two patients reached end-stage renal disease. Adverse events were reported in nine cases; among these, meningococcal infection, anaphylaxis, and eculizumab-related infusion reaction were reported among those treated with eculizumab.
Conclusion
This study provided substantial evidence from a Japanese population that the conversion from plasma therapy to eculizumab therapy should be considered in patients with aHUS who show an incomplete response to plasma therapy. In addition, although no new safety events were detected, careful attention should be paid to meningococcal infection, eculizumab-related infusion reactions and allergic reactions with administration of eculizumab.


Similar content being viewed by others
Data availability
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Fremeaux-Bacchi V, Fakhouri F, Gamier A, Bienaime F, Dragon-Durey MA, Ngo S, et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8(4):554–62.
Noris M, Caprioli J, Bresin E, Mosali C, Pianetti G, Gamba S, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844–59.
Fakhouri F, Zuber J, Frémeaux-Bacchi W, Loirat C. Haemolytic uraemic syndrome. Lancet. 2017;390:681–96.
Brodsky RA. Complement in hemolytic anemia. Blood. 2015;126(22):2459–65.
Afshar-Kharghan V. Atypical hemolytic uremic syndrome. Hematol Am Soc Hematol Educ Progr. 2016;2016(1):217–25.
Ardissino G, Longhi S, Porcaro L, et al. Risk of atypical HUS among family members of patient carrying complement regulatory gene abnormality. KI report. 2021;6:1614–21.
Ariceta G, Besbas N, Johnson S, Karpman D, Landau D, Licht C, et al. Guideline for the investigation and initial therapy of diarrhea-negative hemolytic uremic syndrome. Pediatr Nephrol. 2009;24:687–96.
Johnson S, Stojanovic J, Ariceta G, Bitzan M, Besbas N, Frieliing M, et al. An audit analysis of a guideline for the investigation and initial therapy of diarrhea negative (atypical) hemolytic uremic syndrome. Pediatr Nephrol. 2014;29(10):1967–78.
Licht C, Greenbaum LA, Muus P, Babu S, Bedrosian CL, Cohen DJ, et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87(5):1061–73.
Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Eng J Med. 2013;368:2169–81.
Fakhouri F, Houmant M, Campistol JM, Cataland SR, Espinosa M, Gaber AO, et al. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm. Open-label trial Am J Kidney Dis. 2016;68(1):84–93.
Greenbaum LA, Fila M, Ardissino G, Al-Akash SI, Evans J, Henning P, et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016;89(3):701–11.
Kato H, Miyakawa Y, Hidaka Y, Inoue N, Ito S, Kagami S, et al. Safety and effectiveness of eculizumab for adult patients with atypical hemolytic-uremic syndrome in Japan: interim analysis pf post-marketing surveillance. Clin Exp Nephrol. 2019;23(1):65–75.
Ito S, Hidaka Y, Inoue N, Kaname S, Kato H, Matsumoto M, et al. Safety and effectiveness of eculizumab for pediatric patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance. Clin Exp Nephrol. 2019;23:112–21.
Ito S, Hataya H, Ashida A, Hamada R, Ishikawa T, Ishikawa Y, et al. Eculizumab for paediatric patients with atypical haemolytic-uremic syndrome: full dataset analysis of post-marketing surveillance in Japan. Nephrol Dial TranspL. 2022. https://doi.org/10.1093/ndt/gfac150 (In press).
Kato H, Nangaku M, Hataya H, Sawai T, Ashida A, Fujimaru R, et al. Clinical guides for atypical hemolytic uremic syndrome in Japan. Clin Exp Nephrol. 2016;20:536–43.
Ashida A, Matsumura H, Sawai T, Fujimaru R, Fujii Y, Shirasu A, et al. Clinical features in a series of 258 Japanese pediatric patients with thrombotic microangiopathy. Clin Exp Nephrol. 2018;22:924–30.
Uemura O, Honda M, Matsuyama T, Ishikura K, Hataya H, Yata N, et al. Age, gender, and body length effects on reference serum creatinine levels determined by an enzymatic method in Japanese children: a multicenter study. Clin Exp Nephrol. 2011;15:694–9.
Uemura O, Ishikura K, Gotoh Y, Honda M. Creatinine-based estimated glomerular filtration rate for children younger than 2 years. Clin Exp Nephrol. 2018;22:483–4.
Uemura O, Nagai T, Ishikura K, Ito S, Hataya H, Gotoh Y, et al. Creatinine-based equation to estimate the glomerular filtration rate in Japanese children and adolescents with chronic kidney disease. Clin Exp Nephrol. 2014;18:626–33.
Loirat C, Fakhouri F, Ariceta G, Besbas N, Bitzan M, Bjerre A, et al. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31:15–39.
Goodship THJ, Cook HT, Fakhouri F, Fervenza FC, Frémeaux-Bacchi V, Kavanagh D, et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) controversies conference. Kidney Int. 2017;91(3):539–51.
McNamara LA, Topaz N, Wang X, Hariri S, Fox L, MacNeil JR. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb Mortal Wkly Rep. 2017;66(27):734–7.
Socié G, Caby-Tosi MP, Marantz JL, Cole A, Bedrosian CL, Gasteyger C, et al. Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome: 10-year pharmacovigilance analysis. Br J Haematol. 2019;185(2):297–310.
Acknowledgements
Alexion, AstraZeneca Rare Disease was involved in the study design and provided a formal review of the publication. All of authors retain control and final authority of publication content and decisions, including the choice of journal. The authors thank all patients and physicians who participated in this study.
Funding
This study was funded by Alexion GK.
Author information
Authors and Affiliations
Contributions
AA and AS: designed the study. AA, HM, YF, and SY: collected and analyzed the clinical information and drafted the manuscript. All authors critically read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Employment: Akihiko Shimono (Alexion Pharma GK), Stock options: Akihiko Shimono (Alexion, AstraZeneca Rare Disease), Advisory role: Akira Ashida (Alexion Pharma GK), Honoraria: Akira Ashida (Alexion Pharma GK), Grants Received: Akira Ashida (Alexion Pharma GK,).
Ethical approval
The survey was conducted in accordance with the ethical principles contained within the 1964 Declaration of Helsinki, and the ethical guidelines for epidemiological studies issued by the Ministry of Health, Labour and Welfare, Japan. The survey was approved by the ethics board of Osaka Medical College (approved number: 2675–2) before the study commenced.
Informed consent
For this type of study, formal consent was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ashida, A., Matsumura, H., Shimono, A. et al. Comparison of outcomes after plasma therapy or eculizumab in pediatric patients with atypical hemolytic uremic syndrome. Clin Exp Nephrol 27, 161–170 (2023). https://doi.org/10.1007/s10157-022-02293-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-022-02293-y