Abstract
Background
Several large population-based studies have demonstrated that urinary salt excretion (USALT) is associated with albuminuria. However, this relationship has not been assessed in a large cohort study of patients with chronic kidney disease (CKD). Thus, the present study aimed to elucidate whether USALT was independently associated with albuminuria in a large cohort of patients with CKD.
Methods
This cross-sectional study was conducted in 4075 patients with CKD not on dialysis. USALT (g/day) was estimated from spot urine. Patients were divided into quartiles (Q1–Q4) according to estimated USALT. Multivariable regression models were used to determine whether USALT was independently related to urinary albumin-to-creatinine ratio (UACR) or the presence of macroalbuminuria.
Results
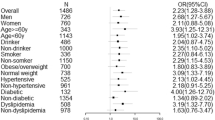
In multivariable linear regression analyses, 1-g/day increment in USALT was significantly associated with log UACR [coefficient 0.098, 95% confidence interval (CI) 0.075–0.121]. In addition, compared with the first USALT quartile, the third and fourth quartiles exhibited significant associations with log UACR (Q3: coefficient 0.305, 95% CI 0.154–0.456; Q4: coefficient 0.601, 95% CI 0.447–0.756). Furthermore, multivariable logistic regression analyses showed that USALT (1-g/day increment) was significantly associated with the presence of macroalbuminuria [odds ratio (OR) 1.11, 95% CI 1.07–1.14]; the third and fourth USALT quartiles exhibited significantly greater risks of macroalbuminuria, compared with the first quartile (Q3: OR 1.33, 95% CI 1.09–1.62; Q4: OR 1.89, 95% CI 1.54–2.32).
Conclusions
This significant association of USALT with UACR and macroalbuminuria suggests that higher USALT may cause increased albuminuria, thereby contributing to kidney disease progression.



Similar content being viewed by others
References
Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc. 2013;88:987–95.
World Health Organization. WHO Guideline: sodium intake for adults and children. Geneva: World Health Organization, 2012. http://www.who.int/nutrition/publications/guidelines/sodium_intake/en/. Accessed 1 Jul 2020.
Powles J, Fahimi S, Micha R, et al. Global burden of diseases nutrition and chronic diseases expert group (NutriCoDE) Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3:e003733.
The Ministry of Health, Labour, and Welfare. The results of the 2017 National Health and Nutrition Survey in Japan. https://www.mhlw.go.jp/content/10904750/000351576.pdf. Accessed 1 Jul 2020.
Mills KT, Chen J, Yang W, et al. Chronic Renal Insufficiency Cohort (CRIC). Study Investigators Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease. JAMA. 2016;315:2200–10.
He J, Mills KT, Appel LJ, et al. Chronic Renal Insufficiency Cohort Study Investigators. Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol. 2016;27:1202–12.
Kidney Disease: Improving Global Outcomes (KDIGO). Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337–414.
Japanese Society of Nephrology. Evidence-based practice guideline for the treatment of CKD. Nihon Jinzo Gakkai Shi. 2018;60:1037–193 (in Japanese).
Hu J, Wang Y, Song N, et al. Estimating 24-hour urinary sodium excretion from spot urine samples in chronic kidney disease patients. J Ren Nutr. 2020;30:11–21.
Nomura K, Asayama K, Jacobs L, et al. Renal function in relation to sodium intake: a quantitative review of the literature. Kidney Int. 2017;92:67–78.
Garofalo C, Borrelli S, Provenzano M, et al. Dietary salt restriction in chronic kidney disease: a meta-analysis of randomized clinical trials. Nutrients. 2018;10:E732.
Han SY, Hong JW, Noh JH, et al. Association of the estimated 24-h urinary sodium excretion with albuminuria in adult Koreans: the 2011 Korea national health and nutrition examination survey. PLoS ONE. 2014;9:e109073.
Verhave JC, Hillege HL, Burgerhof JG, et al. PREVEND Study Group Sodium intake affects urinary albumin excretion especially in overweight subjects. J Intern Med. 2004;256:324–30.
Fox CS, Larson MG, Hwang SJ, et al. Cross-sectional relations of serum aldosterone and urine sodium excretion to urinary albumin excretion in a community-based sample. Kidney Int. 2006;69:2064–9.
Daviglus ML, Greenland P, Stamler J, et al. Relation of nutrient intake to microalbuminuria in nondiabetic middle-aged men and women: International population study on macronutrients and blood pressure (INTERMAP). Am J Kidney Dis. 2005;45:256–66.
Nerbass FB, Pecoits-Filho R, McIntyre NJ, et al. High sodium intake is associated with important risk factors in a large cohort of chronic kidney disease patients. Eur J Clin Nutr. 2015;69:786–90.
Tanaka S, Ninomiya T, Fujisaki K, et al. Fukuoka Kidney disease Registry (FKR) Study Collaboration Group. The Fukuoka Kidney disease Registry (FKR) Study: design and methods. Clin Exp Nephrol. 2017;21:465–73.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO. clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2012;2013(3):S1–150.
Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Schwartz GJ, Haycock GB, Edelmann CM Jr, et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–63.
Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–90.
Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089–100.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Tanaka T, Okamura T, Miura K, et al. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97–103.
Kitiyakara C, Chabrashvili T, Chen Y, et al. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol. 2003;14:2775–822.
Wang H, Chen X, Su Y, et al. p47(phox) contributes to albuminuria and kidney fibrosis in mice. Kidney Int. 2015;87:948–62.
Dobrian AD, Schriver SD, Lynch T, et al. Effect of salt on hypertension and oxidative stress in a rat model of diet-induced obesity. Am J Physiol Renal Physiol. 2003;285:F619–F628628.
Matavelli LC, Zhou X, Varagic J, et al. Salt loading produces severe renal hemodynamic dysfunction independent of arterial pressure in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H814–H819819.
Reitsma S, Slaaf DW, Vink H, et al. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–59.
Rabelink TJ, de Zeeuw D. The glycocalyx–linking albuminuria with renal and cardiovascular disease. Nat Rev Nephrol. 2015;11:667–76.
Desideri S, Onions KL, Qiu Y, et al. A novel assay provides sensitive measurement of physiologically relevant changes in albumin permeability in isolated human and rodent glomeruli. Kidney Int. 2018;93:1086–97.
Oberleithner H, Peters W, Kusche-Vihrog K, et al. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Arch. 2011;462:519–28.
Roscioni SS, Lambers Heerspink HJ, de Zeeuw D. Microalbuminuria: target for renoprotective therapy PRO. Kidney Int. 2014;86:40–9.
Inaguma D, Imai E, Takeuchi A, et al. Chronic Kidney Disease Japan Cohort Study Group. Risk factors for CKD progression in Japanese patients: findings from the Chronic Kidney Disease Japan Cohort (CKD-JAC) study. Clin Exp Nephrol. 2017;21:446–56.
van der Velde M, Halbesma N, de Charro FT, et al. Screening for albuminuria identifies individuals at increased renal risk. J Am Soc Nephrol. 2009;20:852–62.
Fan L, Tighiouart H, Levey AS, et al. Urinary sodium excretion and kidney failure in nondiabetic chronic kidney disease. Kidney Int. 2014;86:582–8.
Konta T, Hao Z, Abiko H, et al. Prevalence and risk factor analysis of microalbuminuria in Japanese general population: the Takahata study. Kidney Int. 2006;70:751–6.
Dougher CE, Rifkin DE, Anderson CA, et al. Spot urine sodium measurements do not accurately estimate dietary sodium intake in chronic kidney disease. Am J Clin Nutr. 2016;104:298–305.
Umemura S, Arima H, Arima S, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
Imai E, Yasuda Y, Horio M, et al. Validation of the equations for estimating daily sodium excretion from spot urine in patients with chronic kidney disease. Clin Exp Nephrol. 2011;15:861–7.
Tamaki J, Kikuchi Y, Yoshita K, et al. HIPOP-OHP Research Group. Stages of change for salt intake and urinary salt excretion: baseline results from the High-Risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) study. Hypertens Res. 2004;27:157–66.
Ogura M, Kimura A, Takane K, et al. Estimation of salt intake from spot urine samples in patients with chronic kidney disease. BMC Nephrol. 2012;13:36.
Acknowledgements
The authors thank the participants in the FKR Study, the members of the FKR Study Group listed below, and all personnel in participating institutions involved in the study. The authors also thank Ryan Chastain-Gross, Ph.D., from Edanz Group (https://en-author-services.edanzgroup.com) for editing a draft of this manuscript. Steering Committee and Principal Collaborators of the FKR Study Group: Satoru Fujimi (Fukuoka Renal Clinic), Hideki Hirakata (Fukuoka Renal Clinic), Tadashi Hirano (Hakujyuji Hospital), Tetsuhiko Yoshida (Hamanomachi Hospital), Takashi Deguchi (Hamanomachi Hospital), Hideki Yotsueda (Harasanshin Hospital), Kiichiro Fujisaki (Iizuka Hospital), Keita Takae (Japanese Red Cross Fukuoka Hospital), Koji Mitsuiki (Japanese Red Cross Fukuoka Hospital), Akinori Nagashima (Japanese Red Cross Karatsu Hospital), Ritsuko Katafuchi (Kano Hospital), Hidetoshi Kanai (Kokura Memorial Hospital), Kenji Harada (Kokura Memorial Hospital), Tohru Mizumasa (Kyushu Central Hospital), Takanari Kitazono (Kyushu University), Toshiaki Nakano (Kyushu University), Toshiharu Ninomiya (Kyushu University), Kumiko Torisu (Kyushu University), Akihiro Tsuchimoto (Kyushu University), Shunsuke Yamada (Kyushu University), Hiroto Hiyamuta (Kyushu University), Shigeru Tanaka (Kyushu University), Dai Matsuo (Munakata Medical Association Hospital), Yusuke Kuroki (National Fukuoka-Higashi Medical Center), Hiroshi Nagae (National Fukuoka-Higashi Medical Center), Masaru Nakayama (National Kyushu Medical Center), Kazuhiko Tsuruya (Nara Medical University), Masaharu Nagata (Shin-eikai Hospital), Taihei Yanagida (Steel Memorial Yawata Hospital), Shotaro Onaka (Tagawa Municipal Hospital).
Author information
Authors and Affiliations
Contributions
AF and MN contributed to research idea, study design and the data interpretation, and wrote the manuscript. ST contributed to the data acquisition and data interpretation. YM and RY contributed to the data interpretation. TN, KT, and TK contributed to the data interpretation and reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at the institutes where the studies were conducted and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Clinical Research Ethics Committee of the Institutional Review Board at Kyushu University (approval number 469–04) and the ethics committees at all participating institutions; it was also registered in the UMIN Clinical Trials Registry (UMIN000007988).
Informed consent
Informed written consent was obtained by all the participants included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Fukui, A., Nakayama, M., Tanaka, S. et al. Association between urinary salt excretion and albuminuria in Japanese patients with chronic kidney disease: the Fukuoka kidney disease registry study. Clin Exp Nephrol 25, 9–18 (2021). https://doi.org/10.1007/s10157-020-01950-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-020-01950-4