Abstract
Background
Glomerular podocyte-derived vascular endothelial growth factor (VEGF) is indispensable for the migration and proliferation of glomerular endothelial cells. In contrast, podocyte-specific Vegf overexpression leads to the collapse of glomerular tufts; however, the mechanisms underlying this outcome have not yet been reported.
Methods
To further clarify the effects of elevated levels of Vegf expression on glomerular cells, we established a dual transgenic mouse line in which Vegf was exclusively and inducibly expressed in podocytes under the control of the “Tet-on system” (Podocin-rtTA/TetO-Vegf164 mice).
Results
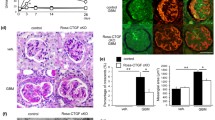
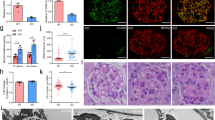
Macroscopic and microscopic examination of Podocin-rtTA/TetO-Vegf164 animals following Vegf induction identified the presence of prominent red bloody spots. In addition, the endothelial cell number was increased along with enlargement of the subendothelial spaces. We also observed impaired endothelial fenestrations and aberrant plasmalemmal vesicle-associated protein-1 (PV-1) expression. In contrast, the mesangial cell number markedly decreased, resulting in a glomerular tuft intussusceptive splitting defect. Furthermore, whereas platelet-derived growth factor-B (PDGF-B) expression in the glomerular cells of Podocin-rtTA/TetO-Vegf164 mice was not decreased, phospho-PDGF receptor immunoreactivity in the mesangial cells was significantly decreased when compared to wild-type animals.
Conclusion
Taken together, the results of this study indicated that the upregulation of podocyte VEGF decreased the number of mesangial cells, likely owing to inhibition of PDGF-B-mediated signaling.




Similar content being viewed by others
References
Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–9.
Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–45.
Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–8.
Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–5.
Tufro A, Norwood VF, Carey RM, Gomez RA. Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol. 1999;10:2125–34.
Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–16.
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–34.
Veron D, Reidy KJ, Bertuccio C, Teichman J, Villegas G, Jimenez J, Shen W, Kopp JB, Thomas DB, Tufro A. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int. 2010;77:989–99.
Ueda H, Miyazaki Y, Matsusaka T, Utsunomiya Y, Kawamura T, Hosoya T, Ichikawa I. Bmp in podocytes is essential for normal glomerular capillary formation. J Am Soc Nephrol. 2008;19:685–94.
Shigehara T, Zaragoza C, Kitiyakara C, Takahashi H, Lu H, Moeller M, Holzman LB, Kopp JB. Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol. 2003;14:1998–2003.
Miyazaki Y, Shimizu A, Pastan I, Taguchi K, Naganuma E, Naganuma E, Suzuki T, Hosoya T, Yokoo T, Saito A, Miyata T, Yamamoto M, Matsusaka T. Keap1 inhibition attenuates glomerulosclerosis. Nephrol Dial Transplant. 2014;29:783–91.
Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998;140:947–59.
Ichimura K, Stan RV, Kurihara H, Sakai T. Glomerular endothelial cells form diaphragms during development and pathologic conditions. J Am Soc Nephrol. 2008;19:1463–71.
Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–60.
Kikkawa Y, Virtanen I, Miner JH. Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane. J Cell Biol. 2003;161:187–96.
Lindahl P, Hellstrom M, Kalen M, Karlsson L, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125:3313–22.
Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–13.
Veron D, Reidy K, Marlier A, Bertuccio C, Villegas G, Jimenez J, Kashgarian M, Tufro A. Induction of podocyte VEGF164 overexpression at different stages of development causes congenital nephrosis or steroid-resistant nephrotic syndrome. Am J Pathol. 2010;177:2225–33.
Satchell SC, Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2009;296:F947–56.
Sorensson J, Fierlbeck W, Heider T, Schwarz K, Park DS, Mundel P, Lisanti M, Ballermann BJ. Glomerular endothelial fenestrae in vivo are not formed from caveolae. J Am Soc Nephrol. 2002;13:2639–47.
Yamamoto I, Horita S, Takahashi T, Tanabe K, Fuchinoue S, Teraoka S, Hattori M, Yamaguchi Y. Glomerular expression of plasmalemmal vesicle-associated protein-1 in patients with transplant glomerulopathy. Am J Transplant. 2007;7:1954–60.
Schlondorff D. The glomerular mesangial cell: an expanding role for a specialized pericyte. FASEB J. 1987;1:272–81.
Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–5.
Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–87.
Frank S, Stallmeyer B, Kampfer H, Schaffner C, Pfeilschifter J. Differential regulation of vascular endothelial growth factor and its receptor fms-like-tyrosine kinase is mediated by nitric oxide in rat renal mesangial cells. Biochem J. 1999;338(Pt 2):367–74.
Boyle SC, Liu Z, Kopan R. Notch signaling is required for the formation of mesangial cells from a stromal mesenchyme precursor during kidney development. Development. 2014;141:346–54.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research (C) (Grant number: 23591202 to Y. M.) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (MEXT). We thank Yamato Kikkawa (Tokyo University of Pharmacy and Life Sciences) for providing the anti-collagen IV antibodies and anti-laminin antibodies. We thank Mitsuko Tamatsukuri and Moeno Ishida for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflicts of interest exist.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (approval number: 24-004).
Informed consent
This section is not applicable to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10157_2017_1450_MOESM1_ESM.pdf
Supplementary material 1 (PDF 1533 kb) Supplementary Fig. 1. Histological comparison of kidney tissues between wild-type and Podocin-rtTA single transgenic mice at P0. (a–h) There were no appreciable differences between wild-type and Podocin-rtTA mice in staining with hematoxylin and eosin (a, b); anti-α-smooth muscle actin (αSMA), a marker for mesangial cells (c, d); CD31 (e, f), a marker for endothelial cells; and anti-Wilm’s tumor (WT-1), a marker for podocytes (g, h). (i–l) Paraformaldehyde-fixed paraffin-embedded specimens were reprocessed for the electron microscopy. Ultrastructures of the basement membrane (depicted by * in k, l), epithelial foot process (fp), and endothelial fenestration (black arrowhead) were intact in both mouse lines. Magnification: 200 × (a–h), scale bars = 10 μm in (i, j) and 500 nm in (k, l)
10157_2017_1450_MOESM2_ESM.pdf
Supplementary material 2 (PDF 369 kb) Supplementary Fig. 2. Component structures of the glomerular basement membrane from wild-type (WT) and Podocin-rtTA/TetO-Vegf164 (Tg) mice. Representative immunostaining for collagen IVα4 (a, b), laminin α5 (c, d), and laminin β2 (e, f) of WT (a, c, and e) and Tg (b, d, and f) mice at P0. Magnification: 400×. Rabbit anti-collagen IVα4 and rabbit anti-laminin α5 and β2 antibodies were kindly provided by Mr. Yamato Kikkawa (School of Pharmacy, Tokyo University of Pharmacy and Life Science, Tokyo, Japan) [15]
About this article
Cite this article
Suyama, M., Miyazaki, Y., Matsusaka, T. et al. Forced expression of vascular endothelial growth factor-A in podocytes decreases mesangial cell numbers and attenuates endothelial cell differentiation in the mouse glomerulus. Clin Exp Nephrol 22, 266–274 (2018). https://doi.org/10.1007/s10157-017-1450-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-017-1450-5