Abstract
Background
Hypouricemia is pathognomonic in syndrome of inappropriate secretion of antidiuretic hormone (SIADH) but the underlying mechanism remains unclear. Based on the previous studies, we hypothesized that V1a receptor may play a principal role in inducing hypouricemia in SIADH and examined uric acid metabolism using a rat model.
Methods
Terlipressin (25 ng/h), a selective V1a agonist, was subcutaneously infused to 7-week-old male Wistar rats (n = 9). Control rats were infused with normal saline (n = 9). The rats were sacrificed to obtain kidney tissues 3 days after treatment. In addition to electrolyte metabolism, changes in expressions of the urate transporters including URAT1 (SLC22A12), GLUT9 (SLC2A9), ABCG2 and NPT1 (SLC17A1) were examined by western blotting and immunohistochemistry.
Results
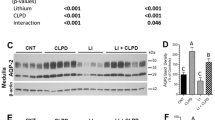
In the terlipressin-treated rats, serum uric acid (UA) significantly decreased and the excretion of urinary UA significantly increased, resulting in marked increase in fractional excretion of UA. Although no change in the expression of URAT1, GLUT9 expression significantly decreased whereas the expressions of ABCG2 and NPT1 significantly increased in the terlipressin group. The results of immunohistochemistry corroborated with those of the western blotting. Aquaporin 2 expression did not change in the medulla, suggesting the independence of V2 receptor stimulation.
Conclusion
Stimulation of V1a receptor induces the downregulation of GLUT9, reabsorption urate transporter, together with the upregulation of ABCG2 and NPT1, secretion urate transporters, all changes of which clearly lead to increase in renal UA clearance. Hypouricemia seen in SIADH is attributable to V1a receptor stimulation.





Similar content being viewed by others
References
Beck LH. Hypouricemia in the syndrome of inappropriate secretion of antidiuretic hormone. N Engl J Med. 1979;301(10):528–30.
Maesaka JK, Gupta S, Fishbane S. Cerebral salt-wasting syndrome: does it exist? Nephron. 1999;82(2):100–9.
Maesaka JK, Imbriano LJ, Ali NM, Ilamathi E. Is it cerebral or renal salt wasting? Kidney Int. 2009;76(9):934–8.
Fenske W, Maier SK, Blechschmidt A, Allolio B, Stork S. Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. Am J Med. 2010;123(7):652–7.
Dorhout Mees EJ, Blom van Assendelft P, Nieuwenhuis MG. Elevation of uric aicd clearance caused by inappropriate antidiuretic hormone secretion. Acta Med Scand. 1971;189(1–2):69–72.
Prospert F, Soupart A, Brimioulle S, Decaux G. Evidence of defective tubular reabsorption and normal secretion of uric acid in the syndrome of inappropriate secretion of antidiuretic hormone. Nephron. 1993;64(2):189–92.
Decaux G, Namias B, Gulbis B, Soupart A. Evidence in hyponatremia related to inappropriate secretion of ADH that V1 receptor stimulation contributes to the increase in renal uric acid clearance. J Am Soc Nephrol. 1996;7(5):805–10.
Wright AF, Rudan I, Hastie ND, Campbell H. A ‘complexity’ of urate transporters. Kidney Int. 2010;78(5):446–52.
Anzai N, Jutabha P, Amonpatumrat-Takahashi S, Sakurai H. Recent advances in renal urate transport: characterization of candidate transporters indicated by genome-wide association studies. Clin Exp Nephrol. 2012;16(1):89–95.
Sakurai H. Urate transporters in the genomic era. Curr Opin Nephrol Hypertens. 2013;22(5):545–50.
Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem. 2004;279(16):16229–36.
Preitner F, Bonny O, Laverriere A, Rotman S, Firsov D, Da Costa A, et al. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci USA. 2009;106(36):15501–6.
Huls M, Brown CD, Windass AS, Sayer R, van den Heuvel JJ, Heemskerk S, et al. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73(2):220–5.
Chiba T, Matsuo H, Kawamura Y, Nagamori S, Nishiyama T, Wei L, et al. NPT1/SLC17A1 is a renal urate exporter in humans and its common gain-of-function variant decreases the risk of renal underexcretion gout. Arthritis Rheumatol. 2015;67(1):281–7.
Yasuoka Y, Kobayashi M, Sato Y, Zhou M, Abe H, Okamoto H, et al. The intercalated cells of the mouse kidney OMCD(is) are the target of the vasopressin V1a receptor axis for urinary acidification. Clin Exp Nephrol. 2013;17(6):783–92.
Burnatowska-Hledin MA, Spielman WS. Vasopressin V1 receptors on the principal cells of the rabbit cortical collecting tubule. Stimulation of cytosolic free calcium and inositol phosphate production via coupling to a pertussis toxin substrate. J Clin Invest. 1989;83(1):84–9.
Ando Y, Tabei K, Asano Y. Luminal vasopressin modulates transport in the rabbit cortical collecting duct. J Clin Invest. 1991;88(3):952–9.
Terada Y, Tomita K, Nonoguchi H, Yang T, Marumo F. Different localization and regulation of two types of vasopressin receptor messenger RNA in microdissected rat nephron segments using reverse transcription polymerase chain reaction. J Clin Invest. 1993;92(5):2339–45.
Tashima Y, Kohda Y, Nonoguchi H, Ikebe M, Machida K, Star RA, et al. Intranephron localization and regulation of the V1a vasopressin receptor during chronic metabolic acidosis and dehydration in rats. Pflugers Arch. 2001;442(5):652–61.
Carmosino M, Brooks HL, Cai Q, Davis LS, Opalenik S, Hao C, et al. Axial heterogeneity of vasopressin-receptor subtypes along the human and mouse collecting duct. Am J Physiol Renal Physiol. 2007;292(1):F351–60.
Jung KY, Endou H. A novel vasopressin receptor in rat early proximal tubule. Biochem Biophys Res Commun. 1991;180(1):131–7.
Birumachi J, Hiroyama M, Fujiwara Y, Aoyagi T, Sanbe A, Tanoue A. Impaired arginine-vasopressin-induced aldosterone release from adrenal gland cells in mice lacking the vasopressin V1A receptor. Eur J Pharmacol. 2007;566(1–3):226–30.
Aoyagi T, Izumi Y, Hiroyama M, Matsuzaki T, Yasuoka Y, Sanbe A, et al. Vasopressin regulates the renin-angiotensin-aldosterone system via V1a receptors in macula densa cells. Am J Physiol Renal Physiol. 2008;295(1):F100–7.
Koshimizu TA, Nakamura K, Egashira N, Hiroyama M, Nonoguchi H, Tanoue A. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev. 2012;92(4):1813–64.
Diamond H, Meisel A. Influence of volume expansion, serum sodium, and fractional excretion of sodium on urate excretion. Pflugers Arch. 1975;356(1):47–57.
Maesaka JK. An expanded view of SIADH, hyponatremia and hypouricemia. Clin Nephrol. 1996;46(2):79–83.
Chino Y, Samukawa Y, Sakai S, Nakai Y, Yamaguchi J, Nakanishi T, et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. 2014;35(7):391–404.
Acknowledgments
We thank all the doctors in the Division of Nephrology, the Department of Internal Medicine, Teikyo University School of Medicine for their continued cooperation. We are especially indebted to Ms. Hiromi Yamaguchi and Ms. Miyuki Fukazawa for their excellent technical assistance. We also thank Dr. Makoto Hosoyamada, MD, PhD, Human Physiology and Pathology, Faculty of Pharma Sciences, Teikyo University, Tokyo. Japan, for discussing on the uric acid measurement and Dr. Hirotaka Matsuo MD, PhD, Department of Integrative Physiology and Bio-Nano Medicine, National Defense Medical College, Tokorozawa, Japan, for discussing on the antibody for urate transporters. This study was supported in part by a Grant-in-Aid for Progressive Renal Diseases Research, Research on Rare and Intractable Disease, from the Ministry of Health, Labour and Welfare of Japan (to SU) and Gout Research Foundation (to SU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have declared that no conflict of interest exists.
About this article
Cite this article
Taniguchi, K., Tamura, Y., Kumagai, T. et al. Stimulation of V1a receptor increases renal uric acid clearance via urate transporters: insight into pathogenesis of hypouricemia in SIADH. Clin Exp Nephrol 20, 845–852 (2016). https://doi.org/10.1007/s10157-016-1248-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-016-1248-x