Abstract
Background
Triplet therapy, androgen receptor signaling inhibitors (ARSIs) plus docetaxel plus androgen-deprivation therapy (ADT), is a novel guideline-recommended treatment for metastatic hormone-sensitive prostate cancer (mHSPC). However, the optimal selection of the patient most likely to benefit from triplet therapy remains unclear.
Methods
We performed a systematic review, meta-analysis, and network meta-analysis to assess the oncologic benefit of triplet therapy in mHSPC patients stratified by disease volume and compare them with doublet treatment regimens. Three databases and meeting abstracts were queried in March 2023 for randomized controlled trials (RCTs) evaluating patients treated with systemic therapy for mHSPC stratified by disease volume. Primary interests of measure were overall survival (OS). We followed the PRISMA guideline and AMSTAR2 checklist.
Results
Overall, eight RCTs were included for meta-analyses and network meta-analyses (NMAs). Triplet therapy outperformed docetaxel plus ADT in terms of OS in both patients with high-(pooled HR: 0.73, 95%CI 0.64–0.84) and low-volume mHSPC (pooled HR: 0.71, 95%CI 0.52–0.97). There was no statistically significant difference between patients with low- vs. high-volume in terms of OS benefit from adding ARSI to docetaxel plus ADT (p = 0.9). Analysis of treatment rankings showed that darolutamide plus docetaxel plus ADT (90%) had the highest likelihood of improved OS in patients with high-volume disease, while enzalutamide plus ADT (84%) had the highest in with low-volume disease.
Conclusions
Triplet therapy improves OS in mHSPC patients compared to docetaxel-based doublet therapy, irrespective of disease volume. However, based on treatment ranking, triplet therapy should preferably be considered for patients with high-volume mHSPC while those with low-volume are likely to be adequately treated with ARSI + ADT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment landscape of metastatic hormone-sensitive prostate cancer (mHSPC) is rapidly changing [1,2,3,4]. Adding an androgen receptor signaling inhibitor (ARSI) with or without docetaxel to androgen deprivation therapy (ADT) is the current guideline-recommended therapy for mHSPC [5]. A recent meta-analysis showed a survival benefit of triplet therapy, consisting of ARSI plus docetaxel plus ADT, compared to ARSI or docetaxel-based doublet regimens; this benefit seemed more prominent in patients with high-volume disease [2]. Metastatic disease burdens, such as high- and low-volume disease, as proposed in the CHAARTED trial, have been widely adopted in clinical trial design, guidelines, and daily practice as it affects the disease state as well as biological and clinical behavior of the heterogeneity in mHSPC [1, 5,6,7]. Recently, results from subgroup analysis stratified by disease volume have been reported with comparative hazard ratios (HRs) for overall survival (OS) between patients with high- and low-volume mHSPC [8]. However, due to the small number of patients resulting in low statistical power due to the small number of patients, the efficacy of triplet therapy in patients with low-volume disease remains inconclusive [2, 8]. Therefore, we conducted this updated systematic review, meta-analysis, and network meta-analysis (NMA) to analyze OS effect of triplet therapy and compare its efficacy to doublet regimens in mHSPC patients according to disease volume.
Methods
The protocol has been registered in the International Prospective Register of Systematic Reviews database (PROSPERO: CRD 404191). This meta-analysis and NMA were conducted based on the guidelines of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement and AMSTAR2 checklist (Supplementary Table 1 and Supplementary Appendix 1) [9, 10].
Search strategy
A literature search on PubMed®, Web of Science™, and Scopus® databases was carried out to identify studies investigating the oncologic outcomes of systemic therapy for mHSPC in March 2023. The detailed search strategy is shown in Supplementary Appendix 2. We also looked for updates on ongoing trials and unpublished randomized controlled trials (RCTs) in abstracts presented at recent major conferences including the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO). The primary outcome of interest was OS. Initial screening based on the titles and abstracts was done by two investigators to identify eligible studies. Potentially relevant studies were subjected to a full-text review. To find additional studies of interest, manual searches of reference lists of relevant articles were also carried out. Disagreements were settled by consensus with co-authors.
Inclusion and exclusion criteria
Studies were considered eligible if they examined patients with mHSPC (Patients), who were treated with triplet therapy (Interventions) and compared to those treated with other currently available treatment regimens (Comparisons), to assess the differential effects of treatment on OS stratified by disease volume (Outcome) only in RCTs (Study design). Studies lacking original patient data, reviews, letters, editorial comments, replies from authors, case reports, and articles not written in English were excluded. In cases of duplicate cohorts, the higher quality or the most recent publication was selected. References of all papers included were scanned for additional studies of interest.
Data extraction
Data were extracted independently by two authors. The first author’s name, publication year, inclusion criteria, agents, agent dosage, number of patients, patient age, the number of patients with de novo disease, the number of patients stratified by disease volume, number of patients treated with docetaxel, and follow-up periods were extracted. Subsequently, the HRs and 95% confidence intervals (CIs) from Cox regression models for OS were retrieved. All discrepancies were resolved by consensus with co-authors.
Risk of bias assessment
Assessment of study quality and risk of bias was carried out using the Cochrane Handbook for Systematic Reviews of Interventions risk-of-bias tool (RoB version 2) (Supplementary Fig. 1) [11]. The risk-of-bias assessment of each study was performed independently by two authors.
Statistical analyses
Meta-analysis
Forest plots with HRs were utilized to analyze the association between systemic therapy and OS. Subgroup analyses were performed in the patients with high- vs. low-volume disease. An additional analysis was conducted in patients with de novo mHSPC. High-volume disease was defined in the CHAARTED trial as the presence of visceral metastases, or four or more bone metastases, of which at least one must be located outside the vertebral column or pelvic bone [7, 12]. Because of homogeneity across international phase 3 RCTs, fixed-effect model was used for calculations of HRs[13]. Heterogeneity among the outcomes of included studies in this meta-analysis was assessed using Cochrane’s Q test. All analyses were conducted using R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria), and the statistical significance level was set at P < 0.05.
Network meta-analysis
A network meta-analysis using random-effect models with a frequentist approach was carried out for direct and indirect treatment comparisons [14, 15]. In the assessment of OS, contrast-based analyses were applied with estimated differences in the log HR and the standard error calculated from the published HR and 95%CIs [16]. The relative effects were presented as HRs and 95% CIs [14]. In addition, we estimated the relative ranking of the different treatments for each outcome using the surface under the cumulative ranking (SUCRA) [14]. Network plots were utilized to illustrate the connectivity of the treatment networks in terms of OS. All statistical analyses were performed using R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study selection and characteristics
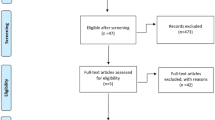
Our initial search identified 832 records. After removing duplicates, 448 records remained for screening of titles and abstracts (Fig. 1). After screening, 415 articles were excluded, and a full-text review of 33 articles/abstracts was performed. According to our inclusion criteria, we finally identified eight RCTs (15 publications and abstracts) comprising 6969 patients eligible for meta-analyses and NMAs [3, 4, 6,7,8, 12, 17,18,19,20,21,22,23,24,25]. In the ARCHES, ENZAMET, and TITAN trials assessing the efficacy of ARSIs plus ADT combinations compared to ADT alone, patients were allowed to use docetaxel at the time of or after randomization[18, 26, 27]. However, only updated results from the ENZAMET trial provided the OS data on patients treated with or without docetaxel, stratified by disease volume[19]. Therefore, we excluded the ARCHES and TITAN trials. The demographics of each included study are shown in Table 1.
Assessment of Risk of bias and quality of study
The risk of bias judgments of each domain for each included study is summarized in Supplementary Fig. 1. All included studies had a low risk of bias owing to the nature of prospective phase 3 RCTs. The quality assessment of this meta-analysis was performed according to the AMSTAR2 checklist; overall confidence in the results of this review was “High” (Supplementary Appendix 1) [10].
Meta‑analysis of triplet therapy vs. docetaxel plus ADT
Three studies comprising 2519 patients provided data on OS in mHSPC patients treated with triplet therapy or docetaxel plus ADT separately for high- and low-volume disease. As shown in Fig. 2, adding ARSI to docetaxel plus ADT improved OS in both patients with high- (pooled HR: 0.73, 95%CI 0.64–0.84) and low- (pooled HR: 0.71, 95%CI 0.52–0.97) volume disease. There was no statistically significant difference between patients with low- vs. high-volume in terms of OS benefit from adding ARSI to docetaxel plus ADT (p = 0.9).
In an analysis focusing on patients with de novo mHSPC, adding ARSI to docetaxel plus ADT improved OS in patients with high-volume disease (pooled HR: 0.72, 95%CI 0.62–0.83), but not in those with low-volume disease (pooled HR: 0.74, 95%CI 0.53–1.01). There was no significant difference in OS between patients with low- vs. high-volume in terms of OS benefit from adding ARSI to docetaxel plus ADT (p = 0.9; Supplementary Fig. 3). The Cochrane’s Q tests revealed no significant heterogeneity in the analysis.
Network meta‑analysis of overall survival among currently available systemic therapies
All eight RCTs including seven different regimens were eligible for this NMA to compare the OS of currently available systemic treatment regimens. The networks of eligible comparisons are graphically described as network plots addressing OS (Supplementary Fig. 2).
Patients with high-volume disease
As shown in Fig. 3, all combinations significantly improved OS compared to ADT alone. Compared to docetaxel plus ADT, darolutamide plus docetaxel plus ADT (HR: 0.69, 95%CI 0.57–0.82) and abiraterone plus docetaxel plus ADT (HR: 0.72, 95%CI 0.55–0.95) improved OS. Compared to abiraterone plus ADT, triplet therapy using darolutamide plus docetaxel plus ADT (HR: 0.81, 95%CI 0.62–1.07) and abiraterone plus docetaxel plus ADT (HR: 0.85, 95%CI 0.60–1.20) did not reach statistical significance in terms of improved OS. Based on the SUCRA analysis of treatment rankings for OS, darolutamide plus docetaxel plus ADT (90%) had the highest likelihood of providing the maximal OS benefit, followed by abiraterone plus docetaxel plus ADT (82%).
Results of NMAs for OS in mHSPC patients with “high-volume” disease treated with systemic therapies; A Forest plots (ADT alone as a comparator), B Forest plots (ABI + ADT as a comparator), C Treatment ranking based on SUCRA graph NMA Network meta-analysis, OS Overall survival, mHSPC metastatic hormone-sensitive prostate cancer, ADT Androgen deprivation therapy, ABI Abiraterone, APA Apalutamide, ARSI Androgen receptor signaling inhibitors, ENZ Enzalutamide, DAR Darolutamide, DOC Docetaxel
Patients with low-volume disease
As shown in Fig. 4, compared to ADT alone, enzalutamide plus ADT (HR: 0.51, 95%CI 0.35–0.75) and abiraterone plus ADT (HR: 0.68, 95%CI 0.50–0.91) improved OS. Despite some evidence to the contrary, all triplet combinations did not reach the conventional level of statistical significance. In addition, compared to docetaxel plus ADT, only enzalutamide plus ADT significantly improved OS (HR: 0.56, 95%CI 0.36–0.87). Considering comparison to abiraterone plus ADT doublet, none of the other combination therapies showed significant improvements. Based on the SUCRA analysis of treatment rankings for OS, enzalutamide plus ADT had the highest likelihood of providing the maximal OS benefit (84%), followed by enzalutamide plus docetaxel plus ADT (75%) and darolutamide plus docetaxel plus ADT (65%).
Results of NMAs for OS in mHSPC patients with “low-volume” disease treated with systemic therapies; A Forest plots (ADT alone as a comparator), B Forest plots (ABI + ADT as a comparator), C Treatment ranking based on SUCRA graph NMA Network meta-analysis, OS Overall survival, mHSPC metastatic hormone-sensitive prostate cancer, ADT Androgen deprivation therapy, ABI Abiraterone, APA Apalutamide, ARSI Androgen receptor signaling inhibitors, ENZ Enzalutamide, DAR Darolutamide, DOC Docetaxel
Discussion
We report an updated meta-analysis and NMA analyzing and comparing the effect of triplet therapy on OS in patients with mHSPC stratified by disease volume. There are several key findings to our study. First, triplet therapy outperformed docetaxel plus ADT in terms of OS in patients with both high- and low-volume disease. Second, treatment ranking analysis confirmed that triplet therapy had the highest likelihood of improved OS among currently available treatment regimens in patients with high-volume disease. Conversely, in low-volume mHSPC, doublet therapy using ARSI (enzalutamide) plus ADT had the highest likelihood of improved OS.
Our meta-analysis demonstrated the OS benefit from triplet therapy even in mHSPC patients with low-volume disease compared to docetaxel plus ADT. Indeed, HRs for OS from each RCT in patients with low-volume disease were all comparable with those with high-volume, while no study showed statistical significant difference potentially due to the low number of patients and events for low-volume disease resulting in a low statistical power (Fig. 4) [3, 8, 19]. Based on this and a previous meta-analysis without the results from a subgroup analysis of the ARASENS trial, ARSI-based doublet therapy is currently recommended for low-volume disease [2, 28]. However, when synthesizing the results from three RCTs, we demonstrated significant OS benefit from triplet therapy compared to docetaxel plus ADT, even in patients with low-volume disease. On the contrary, when focusing on patients with de novo low-volume disease, we found no significant OS benefit from triplet therapy compared to docetaxel plus ADT. A possible explanation is low statistical power based on the low population of this group, highlighting the need for further studies with larger sample sizes to confirm these findings.
Low-volume mHSPC generally has a more favorable disease trajectory and relatively good prognosis when treated with combination therapies; therefore, long-term follow-up with larger sample size is needed to elucidate the survival outcomes. A recent meta-analysis with long-term follow-up (median 6 years) using individual participant data from the GETUG-15, CHAARTED, and STAMPEDE trials showed OS benefit from adding docetaxel to ADT in mHSPC patients, irrespective of disease volume, while the absolute value was more prominent in patients with high- (HR 0.60, 95%CI 0.52–0.68) compared to those with low- (HR 0.78, 95%CI 0.64–0.94) volume disease [29]. Notably, despite including updated results from the ENZAMET trial, which has a median follow-up of 68 months, the follow-up periods in the ARASENS and PEACE-1 trials at 45.7 and 43 months respectively, were not long enough to fully evaluate the OS outcomes in patients with low-volume disease [3, 4, 19]. Extended follow-up periods are needed to confirm a robust OS benefit from triplet therapy in patients with low-volume disease and to identify the best combination for each individual patient.
Our treatment rankings revealed that triplet therapy, darolutamide plus docetaxel plus ADT, had the highest likelihood of improved OS in patients with high-volume disease. This updated result, including subgroup analyses from the ENZAMET and ARASENS trials, was in line with the results from a previous NMA [2]. High-volume disease is generally associated with biologically and clinically aggressive disease bearing a high probability of harboring androgen receptor-independent cells; suggesting a possible rationale for the efficacy of adding cytotoxic chemotherapy to ADT + ARSI for high-volume disease [30]. In agreement with this concept, the OS benefit from adding docetaxel to ADT was most prominent in patients with high-volume disease in the CHAARTED trial [21]. Taken together, the state of current evidence suggests that triplet therapy with docetaxel plus ASRI plus ADT should be considered in patients with high-volume disease who can tolerate it.
In contrast, enzalutamide plus ADT not only had the highest likelihood of improved OS in patients with low-volume mHSPC but also significantly improved OS when compared to docetaxel plus ADT alone. This suggests a limited role for triplet therapy in patients with low-volume disease and it supports individual shared decision-making. However, our analysis should be interpreted with caution due to possible selection bias. In this NMA, we extracted the data on subgroups treated with or without docetaxel from the PEACE-1 and ENZAMET trials. In these trials, the decision of docetaxel application was based on the physician’s discretion. This might have led to selection bias; younger, healthier patients with more aggressive diseases are more likely to receive docetaxel. For example, in the ENZAMET trial, patients who did not receive docetaxel were more likely to harbor low-volume disease; 71% of patients treated with docetaxel harbored high-volume disease, while this rate was only 37% in patients who did not receive docetaxel [19].
Since the publication of the CHAARTED trial results in 2015, docetaxel plus ADT has gained acceptance as a standard of care (SOC) in patients with mHSPC. As mentioned before, this greatly affected the study design/eligibility of RCTs [18, 26, 31]. Unlike the ENZAMET trial, the ARCHES and TITAN trials also allowed the use of docetaxel as SOC; patients treated with docetaxel were included in both treatment arms. This made reliable NMAs including “true” ARSI-based doublet regimens difficult. Therefore, our treatment rankings lack the apalutamide plus ADT with or without docetaxel, not reflecting all the currently available regimens.
In the argument of whether triplet therapy outperforms ARSI-based doublet therapy in terms of survival outcomes, our previous NMA showed that darolutamide plus docetaxel plus ADT outperformed abiraterone plus ADT in terms of OS in the overall cohort[2]. However, present NMAs stratified by disease volume failed to show the statistical OS superiority of triplet therapy over ARSI-based doublet therapy in both patients with high- and low volume disease. A possible explanation is that these results were based on subgroup analyses from each RCT, limiting the statistical power. Only a head-to-head RCT comparing triplet therapy versus ARSI-based doublet therapy will clarify the potential impact on survival outcomes.
Last but not least, the benefit-harm balance is, indeed, an important factor for clinical decision-making. A recent meta-analysis showed that ARSI-based doublet therapy had high probabilities for a net clinical benefit; however, docetaxel-based doublet as well as triplet therapy appeared unlikely to be beneficial [32]. Docetaxel is known to increase the risk of severe adverse events and can reduce the patient quality of life [2, 33]. Our previous meta-analysis confirmed that docetaxel-based combination had a higher likelihood of severe adverse events, with triplet therapies being the highest adverse event rates [2, 33]. In the subgroup analysis of the ARASENS trial, 74% of patients with low-volume disease who received triplet therapy experienced severe adverse events [8]. Although our study showed the oncologic utility of triplet therapy, shared decision-making considering the benefit-harm balance is essential, specifically in such patients with a long life expectancy [8]. Further investigation is needed to select the optimal candidates who are most likely to benefit from triplet therapy.
Besides the abovementioned issues, the current study has several limitations that need to be considered. First, despite careful data extraction from RCTs, each study differed in patient proportion, such as the rates of de novo/metachronous and high/low-volume disease. Our analyses based on subgroup analyses of each RCT might reduce the patient heterogeneity; however, this differential proportion could potentially affect the outcomes. Second, in the ARCHES, ENZAMET, and TITAN trials assessing the efficacy of ARSIs plus ADT combinations compared to ADT alone, patients were allowed to use docetaxel at the time of or after randomization [18, 26, 27]. However, only updated results from the ENZAMET trial provided the OS data on patients treated with or without docetaxel, stratified by disease volume [19]. Therefore, we excluded the ARCHES and TITAN trials. Third, NMAs have a limited role in facilitating patient selection; this approach cannot substitute for a direct comparison of each treatment and is mostly hypothesis-generating. Additionally, while patients with low-volume disease are generally less likely to experience mortality in the short-term follow-up, the effectiveness of triplet therapy may not be apparent in our analysis. Our findings need to be validated in head-to-head, well-designed RCTs. Fourth, our analyses are limited in assessing which regimens are the best combination for each clinical setting. Due to the nature of subgroup analyses, a limited number of studies assessing the outcomes stratified by disease volume. Furthermore, there was a paucity of studies reporting outcomes stratified by metachronous high- or low-volume separately. Fifth, although we investigated OS stratified by disease volume, other factors, such as Gleason pattern 5, TP53 mutations, also affect mortality even for patients with low-volume disease [34, 35]. Therefore, patients with these factors may benefit from triplet therapy even in the context of low-volume disease. Finally, the ENZAMET trial included the use of non-steroidal antiandrogen therapy with ADT in the control arm. This might provide a differential survival benefit in the control arm, therefore, weighing against the survival outcomes of enzalutamide. Again, we need further RCTs, especially for RCTs directly comparing triplet therapy versus ARSI-based doublet therapy. In addition, the comparative efficacy of darolutamide with other ARSIs as ARSI-based doublet combinations from the ongoing ARANOTE trial (NCT04736199) will enrich the treatment option for mHSPC. Based on this analysis and a previous meta-analysis, which did not include results from a subgroup analysis of the AR therapy is currently recommended for patients with low-volume disease [36].
Conclusions
We found that compared to docetaxel plus ADT triplet therapy improves OS in patients with mHSPC, irrespective of disease volume. Treatment ranking revealed that triplet therapy should be a first-choice option in patients with high-volume disease who can tolerate it. However, in patients with low-volume disease, ARSI plus ADT doublet seemed to be the first choice with respect to OS. Further investigation with well-designed RCTs is awaited to clarify the comparative oncologic outcomes between triplet and ARSI-based doublet therapies and select the optimal candidates who are most likely to benefit from triplet therapy.
Abbreviations
- ADT:
-
Androgen deprivation therapy
- ASCO:
-
American Society of Clinical Oncology
- ARSI:
-
Androgen receptor signaling inhibitor
- CI:
-
Confidence interval
- ESMO:
-
European Society for Medical Oncology
- HR:
-
Hazard ratio
- mHSPC:
-
Metastatic hormone-sensitive prostate cancer
- NMA:
-
Network meta-analysis
- OS:
-
Overall survival
- PRISMA:
-
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- RCTs:
-
Randomized controlled trials
- SOC:
-
Standard of care
- SUCRA:
-
Surface under the cumulative ranking
References
Cornford P, van den Bergh RCN, Briers E et al (2021) EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: treatment of relapsing and metastatic prostate cancer. Eur Urol 79:263–282
Yanagisawa T, Rajwa P, Thibault C et al (2022) Androgen receptor signaling inhibitors in addition to docetaxel with androgen deprivation therapy for metastatic hormone-sensitive prostate cancer: a systematic review and meta-analysis. Eur Urol 82:584–598
Fizazi K, Foulon S, Carles J et al (2022) Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 x 2 factorial design. Lancet 30(399):1695–1707
Smith MR, Hussain M, Saad F et al (2022) Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. https://doi.org/10.1016/j.eururo.2022.03.004
Schaeffer EM, Srinivas S, Adra N et al (2022) NCCN Guidelines® Insights: Prostate Cancer, Version 1.2023. J Natl Compr Canc Netw 20:1288–1298
Hoyle AP, Ali A, James ND et al (2019) Abiraterone in “high-” and “low-risk” metastatic hormone-sensitive prostate cancer. Eur Urol 76:719–728
Kyriakopoulos CE, Chen YH, Carducci MA et al (2018) Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 10(36):1080–1087
Hussain M, Tombal B, Saad F et al (2010) Darolutamide plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer by disease volume and risk subgroups in the phase III ARASENS trial. J Clin Oncol 41(20):3595–3607. https://doi.org/10.1200/JCO.23.00041
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 21(6):e1000100
Shea BJ, Reeves BC, Wells G et al (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 21(358):j4008
Higgins JP, Altman DG, Gotzsche PC et al (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 18(343):d5928
Sweeney CJ, Chen YH, Carducci M et al (2015) Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 20(373):737–746
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 6(327):557–560
Shim SR, Kim SJ, Lee J et al (2019) Network meta-analysis: application and practice using R software. Epidemiol Health 41:e2019013
van Valkenhoef G, Lu G, de Brock B et al (2012) Automating network meta-analysis. Res Synth Methods 3:285–299
Woods BS, Hawkins N, Scott DA (2010) Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial. BMC Med Res Methodol 10(10):54
Clarke NW, Ali A, Ingleby FC et al (2019) Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol 1(30):1992–2003
Davis ID, Martin AJ, Stockler MR et al (2019) Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 11(381):121–131
Davis ID, Martin AJ, Zielinski RR et al (2022) Updated overall survival outcomes in ENZAMET (ANZUP 1304), an international, cooperative group trial of enzalutamide in metastatic hormone-sensitive prostate cancer (mHSPC). J Clin Oncol 40:LBA5004-LBA
Fizazi K, Tran N, Fein L et al (2017) Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 27(377):352–360
Fizazi K, Tran N, Fein L et al (2019) Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol 20:686–700
Gravis G, Boher JM, Joly F et al (2016) Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol 70:256–262
Gravis G, Fizazi K, Joly F et al (2013) Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol 14:149–158
James ND, de Bono JS, Spears MR et al (2017) Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 27(377):338–351
James ND, Sydes MR, Clarke NW et al (2016) Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 19(387):1163–1177
Armstrong AJ, Szmulewitz RZ, Petrylak DP et al (2019) ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol 10(37):2974–2986
Chi KN, Chowdhury S, Bjartell A et al (2021) Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol 10(39):2294–2303
Dzimitrowicz HE, Armstrong AJ (2022) Triplet therapy: entering the metaverse of metastatic hormone-sensitive prostate cancer treatment. Eur Urol 82:599–601
Vale CL, Fisher D, Godolphin P et al (2022) Defining more precisely the effects of docetaxel plus ADT for men with mHSPC: meta-analysis of individual participant data from randomized trials. J Clin Oncol 40:5070
Nelson PS (2012) Molecular states underlying androgen receptor activation: a framework for therapeutics targeting androgen signaling in prostate cancer. J Clin Oncol 20(30):644–646
Chi KN, Agarwal N, Bjartell A et al (2019) Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 4(381):13–24
Menges D, Yebyo HG, Sivec-Muniz S et al (2022) Treatments for metastatic hormone-sensitive prostate cancer: systematic review, network meta-analysis, and benefit-harm assessment. Eur Urol Oncol 5:605
Yanagisawa T, Kimura T, Hata K et al (2022) Does castration status affect docetaxel-related adverse events? Identification of risk factors for docetaxel-related adverse events in metastatic prostate cancer. Prostate 82:1322–1330
Warner EW, Van der Eecken K, Murtha AJ et al (2024) Multiregion sampling of de novo metastatic prostate cancer reveals complex polyclonality and augments clinical genotyping. Nat Cancer 5:1–7
Lim B, Lee W, Kyung YS et al (2022) Biopsy-detected Gleason grade 5 tumor is an additional prognostic factor in metastatic hormone-sensitive prostate cancer. J Cancer Res Clin Oncol 148:727–734
Rajwa P, Pradere B, Gandaglia G et al (2022) Intensification of systemic therapy in addition to definitive local treatment in nonmetastatic unfavourable prostate cancer: a systematic review and meta-analysis. Eur Urol 82(1):82–96
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Matsukawa, A., Rajwa, P., Kawada, T. et al. Impact of disease volume on survival efficacy of triplet therapy for metastatic hormone-sensitive prostate cancer: a systematic review, meta-analysis, and network meta-analysis. Int J Clin Oncol 29, 716–725 (2024). https://doi.org/10.1007/s10147-024-02485-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02485-4