Abstract
Background
Granulocyte colony-stimulating factor (G-CSF) is commonly administered to cancer patients undergoing myelosuppressive chemotherapy, especially when incidence rate of febrile neutropenia (FN) surpasses 20%. While primary prophylaxis with G-CSF has been proven effective in preventing FN in patients with cancer, there is limited evidence regarding its efficacy in specifically, lung cancer. Our systematic review focused on the efficacy of G-CSF primary prophylaxis in lung cancer.
Methods
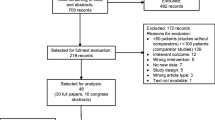
We extracted studies on non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC) using the PubMed, Ichushi Web, and Cochrane Library databases. Two reviewers assessed the extracted studies for each type of lung cancer and conducted quantitative and meta-analyses of preplanned outcomes, including overall survival, FN incidence, infection-related mortality, quality of life, and musculoskeletal pain.
Results
A limited number of studies were extracted: two on NSCLC and six on SCLC. A meta-analysis was not conducted owing to insufficient data on NSCLC. Two case–control studies explored the efficacy of primary prophylaxis with G-CSF in patients with NSCLC (on docetaxel and ramucirumab therapy) and indicated a lower FN frequency with G-CSF. For SCLC, meta-analysis of five studies showed no significant reduction in FN incidence, with an odds ratio of 0.38 (95% confidence interval 0.03–5.56, P = 0.48). Outcomes other than FN incidence could not be evaluated due to low data availability.
Conclusion
Limited data are available on G-CSF prophylaxis in lung cancer. Primary prophylaxis with G-CSF may be weakly recommended in Japanese patients with NSCLC undergoing docetaxel and ramucirumab combination therapy.



Similar content being viewed by others
Data availability
Data associated with this systematic review can be accessed from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/CAAC.21660
Soria J-C, Ohe Y, Vansteenkiste J et al (2018) Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 378:113–125. https://doi.org/10.1056/NEJMOA1713137
Peters S, Camidge DR, Shaw AT et al (2017) Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 377:829–838. https://doi.org/10.1056/NEJMOA1704795
Shaw AT, Ou S-HI, Bang Y-J et al (2014) Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 371:1963–1971. https://doi.org/10.1056/NEJMOA1406766
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 375:1823–1833. https://doi.org/10.1056/NEJMOA1606774
Garassino MC, Gadgeel S, Speranza G et al (2023) Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J Clin Oncol 41:1992–1998. https://doi.org/10.1200/JCO.22.01989
Hellmann MD, Paz-Ares L, Bernabe Caro R et al (2019) Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 381:2020–2031. https://doi.org/10.1056/NEJMOA1910231
De Oliveira BC, Lewis S, Sandschafer D et al (2023) Two decades of pegfilgrastim: what have we learned? Where do we go from here? Curr Med Res Opin 39:707–718. https://doi.org/10.1080/03007995.2023.2196197
Smith TJ, Bohlke K, Lyman GH et al (2015) Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 33:3199–3212. https://doi.org/10.1200/JCO.2015.62.3488
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Hematopoietic Growth Factors (Version 3. 2024). https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf. Accessed 2 Feb 2024
Klastersky J, de Naurois J, Rolston K et al (2016) Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol 27:v111–v118. https://doi.org/10.1093/ANNONC/MDW325
Aapro MS, Bohlius J, Cameron DA et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32. https://doi.org/10.1016/J.EJCA.2010.10.013
Mouri A, Kaira K, Shiono A et al (2019) Clinical significance of primary prophylactic pegylated-granulocyte-colony stimulating factor after the administration of ramucirumab plus docetaxel in patients with previously treated non-small cell lung cancer. Thorac Cancer 10:1005–1008. https://doi.org/10.1111/1759-7714.13022
Takase M, Shibata K, Iwasa K, et al (2019) Measures to Ensure Safety of Docetaxel plus Ramucirumab for Advanced Non-Small-Cell Lung Cancer as the Second- or Later-Line (Article in Japanese). In: Gan To Kagaku Ryoho. https://pubmed.ncbi.nlm.nih.gov/31273171/. Accessed 23 Nov 2023
Schiller JH, Kim K, Hutson P et al (1996) Phase II study of topotecan in patients with extensive-stage small-cell carcinoma of the lung: an Eastern Cooperative Oncology Group Trial. J Clin Oncol 14:2345–2352. https://doi.org/10.1200/JCO.1996.14.8.2345
Goto K, Sekine I, Nishiwaki Y et al (2004) Multi-institutional phase II trial of irinotecan, cisplatin, and etoposide for sensitive relapsed small-cell lung cancer. Br J Cancer 91:659–665. https://doi.org/10.1038/SJ.BJC.6602056
Hata A, Katakami N, Fujita S et al (2011) Amrubicin at a lower-dose with routine prophylactic use of granulocyte-colony stimulating factor for relapsed small-cell lung cancer. Lung Cancer 72:224–228. https://doi.org/10.1016/J.LUNGCAN.2010.08.009
Goto K, Ohe Y, Shibata T et al (2016) Combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second-line treatment for patients with sensitive relapsed small-cell lung cancer (JCOG0605): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 17:1147–1157. https://doi.org/10.1016/S1470-2045(16)30104-8
Yamaguchi T, Kurita Y, Saito R et al (1994) Clinical effect of recombinant human G-CSF on neutropenia induced by chemotherapy in small-cell lung cancer patients. Biotherapy 8:1423–1429
Negoro Y, Yano R, Yoshimura M et al (2019) Influence of UGT1A1 polymorphism on etoposide plus platinum-induced neutropenia in Japanese patients with small-cell lung cancer. Int J Clin Oncol 24:256–261. https://doi.org/10.1007/S10147-018-1358-4
Yoh K, Hosomi Y, Kasahara K et al (2016) A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy. Lung Cancer 99:186–193. https://doi.org/10.1016/J.LUNGCAN.2016.07.019
Acknowledgements
We thank Natsuki Narita for her support of our systematic review. We would like to thank Editage (www.editage.jp) for English language editing.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the conception and design of this study. E.I. wrote the draft of the manuscript, and all authors reviewed and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Eiki Ichihara received honoraria from Eli Lilly and research funding from MSD, Ono Pharmaceutical, Jansen Pharma, and Takeda Pharmaceutical. Go Makimoto received honoraria from Kyowa Kirin. Yukinori Ozaki received honoraria from Daiichi-Sankyo, Pfizer, Chugai, Lilly, and Kyowa Kirin. Kenji Tsuchihashi received honoraria from Ono Pharmaceutical, Chugai Pharmaceutical, Taiho Pharmaceutical, and Novartis Pharma. Yuji Miura received honoraria from Ono Pharmaceutical, MSD, Takeda Pharmaceuticals, Eisai, Bristol Myers Squibb, and research funding from Ono Pharmaceutical and MSD. Shingo Yano received research funding from Otsuka Pharmaceutical. Dai Maruyama received honoraria from Ono Pharmaceutical, Janssen Pharma, Nippon Shinyaku, Eisai, Mundipharma, Kyowa Kirin, Chugai Pharmaceutical, Zenyaku, MSD, SymBio, Sanofi, AbbVie, Takeda Pharmaceutical, AstraZeneca, Bristol Myers Squibb, and Genmab, and research funding from Amgen, Astellas Biopharma, Novartis, Kyowa Kirin, Ono Pharmaceutical, Chugai Pharmaceutical, Janssen Pharma, Takeda Pharmaceutical, Otsuka Pharmaceutical, Sanofi, Astellas, Bristol Myers Squibb, AbbVie, Eisai, MSD, Taiho Pharmaceutical, AstraZeneca, Eli Lilly, and Genmab. Tetsuhiro Yoshinami received honoraria from Kyowa Kirin, Pfizer, Chugai Pharmaceutical, Eli Lilly, MSD, AstraZeneca, and Eizai. Takashi Motohashi received honoraria from AstraZeneca, Chugai Pharmaceutical, and Myriad Genetics. Eishi Baba received honoraria from Chugai Pharmaceutical and Daiichi-Sankyo, and research funding from Taiho Pharmaceutical and Chugai Pharmaceutical. Takahiro Kimura received honoraria from Sanofi. Shinji Nakao received honoraria from Kyowa Kirin. Atsushi Sato received honoraria from Chugai Pharmaceutical, and Taiho Pharmaceutical. Atsushi Sato received research funding from Chugai Pharmaceutical, and Taiho Pharmaceutical. Toshimi Takano received honoraria from Daiichi-Sankyo, Chugai Pharmaceutical, and Eli Lilly. Toshio Kubo received honoraria from Chugai Pharmaceutical.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ichihara, E., Ochi, N., Makimoto, G. et al. Effectiveness and safety of primary prophylaxis with G-CSF for lung cancer: a systematic review and meta-analysis to develop clinical practice guidelines for the use of G-CSF 2022. Int J Clin Oncol 29, 355–362 (2024). https://doi.org/10.1007/s10147-024-02469-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02469-4