Abstract
Objective
The purpose of this study was to compare the clinical outcomes and toxicities between induction chemotherapy (IC) + chemo-radiotherapy (CRT) and CRT alone in patients with locally advanced esophageal squamous cell carcinoma (ESCC), to explore the appropriate thoracic radiotherapy (TRT) timing after IC and to identify prognostic factors.
Methods
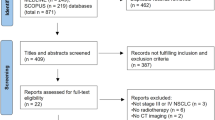
450 ESCC patients were included from September 2011 to December 2020, 238 of whom received IC/CRT. Propensity score matching was performed to balance potential confounders between the two groups. Multivariate Cox regression analysis was used to identify the independent prognostic factors.
Results
Patients who received IC/CRT experienced improved overall survival (OS) (38.5 vs. 28.8 months) and progression-free survival (PFS) (41.0 vs. 22.0 months) before matching, with similar results after matching. In the IC/CRT group, early TRT had more favorable survival than late TRT both matching before and after. In subgroup analysis, early TRT combination concurrent chemotherapy had better OS and PFS than late TRT combination concurrent chemotherapy. In addition, early TRT had better survival benefits regardless of the N stage. Notably, the IC/CRT group and early TRT group had manageable toxicities reaction compared with CRT alone group and the late TRT group. The nomogram was developed to predict the OS and PFS based on multivariate analysis results. The C-index was 0.743 and 0.722, respectively.
Conclusion
IC/CRT and early TRT could yield satisfactory clinical outcomes and controllable toxicities in locally advanced ESCC. The IC plus early concurrent CRT might be a promising treatment strategy for improving further survival in ESCC.






Similar content being viewed by others
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- EC:
-
Esophageal cancer
- EAC:
-
Esophageal adenocarcinoma
- ESCC:
-
Esophageal squamous cell carcinoma
- dCRT:
-
Definitively chemoradiotherapy
- OS:
-
Overall survival
- IC/CRT:
-
Induction chemotherapy before chemoradiotherapy
- CRT:
-
Chemoradiotherapy
- TRT:
-
Thoracic radiotherapy
- EUS:
-
Endoscopic ultrasonography
- CT:
-
Computed tomography
- PET/CT:
-
Positron emission tomography
- TNM:
-
Tumor node metastasis
- GTV:
-
Gross tumor volume
- CTV:
-
Clinical tumor volume
- PTV:
-
Planning tumor volume
- CFRT:
-
Convention fractionate radiotherapy
- PFS:
-
Progression-free survival
- PSM:
-
Propensity score matching
- BMI:
-
Body mass index
- CCRT:
-
Concurrent chemoradiotherapy
- T:
-
Tumor
- N:
-
Nodes
- IPTW:
-
The inverse probability of treatment weighting
References
Sung H et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Chen W et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66:115–132. https://doi.org/10.3322/caac.21338
Waddell T et al (2013) Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 14:481–489. https://doi.org/10.1016/S1470-2045(13)70096-2
Lordick F et al (2013) Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 14:490–499. https://doi.org/10.1016/S1470-2045(13)70102-5
Shah MA et al (2017) Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol 3:620–627. https://doi.org/10.1001/jamaoncol.2016.5580
Ismail A et al (2011) Early G(1) cyclin-dependent kinases as prognostic markers and potential therapeutic targets in esophageal adenocarcinoma. Clin Cancer Res 17:4513–4522. https://doi.org/10.1158/1078-0432.CCR-11-0244
Swisher EM et al (2017) Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol 18:75–87. https://doi.org/10.1016/S1470-2045(16)30559-9
Pennathur A, Gibson MK, Jobe BA et al (2013) Oesophageal carcinoma. Lancet 381:400–412. https://doi.org/10.1016/S0140-6736(12)60643-6
van Hagen P et al (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366:2074–2084. https://doi.org/10.1056/NEJMoa1112088
Cunningham D et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20. https://doi.org/10.1056/NEJMoa055531
Hara H et al (2013) Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci 104:1455–1460. https://doi.org/10.1111/cas.12274
Satake H et al (2016) A prospective, multicenter phase I/II study of induction chemotherapy with docetaxel, cisplatin and fluorouracil (DCF) followed by chemoradiotherapy in patients with unresectable locally advanced esophageal carcinoma. Cancer Chemother Pharmacol 78:91–99. https://doi.org/10.1007/s00280-016-3062-2
Luo LL et al (2017) Comparative outcomes of induction chemotherapy followed by definitive chemoradiotherapy versus chemoradiotherapy alone in esophageal squamous cell carcinoma. J Cancer 8:3441–3447. https://doi.org/10.7150/jca.21131
Minsky BD et al (1999) Final report of Intergroup Trial 0122 (ECOG PE-289, RTOG 90–12): Phase II trial of neoadjuvant chemotherapy plus concurrent chemotherapy and high-dose radiation for squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys 43:517–523. https://doi.org/10.1016/s0360-3016(98)00463-5
De Ruysscher D et al (2006) Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann Oncol 17:543–552. https://doi.org/10.1093/annonc/mdj094
Pijls-Johannesma M et al (2007) Timing of chest radiotherapy in patients with limited stage small cell lung cancer: a systematic review and meta-analysis of randomised controlled trials. Cancer Treat Rev 33:461–473. https://doi.org/10.1016/j.ctrv.2007.03.002
Takada M et al (2002) Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 20:3054–3060. https://doi.org/10.1200/JCO.2002.12.071
Han J, Fu C, Li B (2021) Clinical outcomes of extensive-stage small cell lung cancer patients treated with thoracic radiotherapy at different times and fractionations. Radiat Oncol 16:47. https://doi.org/10.1186/s13014-021-01773-x
Donohoe CL, Phillips AW (2017) Cancer of the esophagus and esophagogastric junction: an 8(th) edition staging primer. J Thorac Dis 9:E282–E284. https://doi.org/10.21037/jtd.2017.03.39
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 46:399–424. https://doi.org/10.1080/00273171.2011.568786
Sudo K et al (2014) Importance of surveillance and success of salvage strategies after definitive chemoradiation in patients with esophageal cancer. J Clin Oncol 32:3400–3405. https://doi.org/10.1200/JCO.2014.56.7156
Day FL et al (2011) Phase I trial of docetaxel, cisplatin and concurrent radical radiotherapy in locally advanced oesophageal cancer. Br J Cancer 104:265–271. https://doi.org/10.1038/sj.bjc.6606051
Adelstein DJ, Leblanc M (2006) Does induction chemotherapy have a role in the management of locoregionally advanced squamous cell head and neck cancer? J Clin Oncol 24:2624–2628. https://doi.org/10.1200/JCO.2005.05.3629
Zhang Y et al (2019) Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med 381:1124–1135. https://doi.org/10.1056/NEJMoa1905287
Gau M, Karabajakian A, Reverdy T et al (2019) Induction chemotherapy in head and neck cancers: results and controversies. Oral Oncol 95:164–169. https://doi.org/10.1016/j.oraloncology.2019.06.015
Liu S et al (2021) Induction chemotherapy followed by definitive chemoradiotherapy versus chemoradiotherapy alone in esophageal squamous cell carcinoma: a randomized phase II trial. Nat Commun 12:4014. https://doi.org/10.1038/s41467-021-24288-1
Haddad RI et al (2018) Induction chemotherapy in locally advanced squamous cell carcinoma of the head and neck: role, controversy, and future directions. Ann Oncol 29:1130–1140. https://doi.org/10.1093/annonc/mdy102
Stahl M et al (2005) Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 23:2310–2317. https://doi.org/10.1200/JCO.2005.00.034
Spiro SG et al (2006) Early compared with late radiotherapy in combined modality treatment for limited disease small-cell lung cancer: a London Lung Cancer Group multicenter randomized clinical trial and meta-analysis. J Clin Oncol 24:3823–3830. https://doi.org/10.1200/JCO.2005.05.3181
Fried DB et al (2004) Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol 22:4837–4845. https://doi.org/10.1200/JCO.2004.01.178
Wong AT et al (2017) Effect of thoracic radiotherapy timing and fractionation on survival in nonmetastatic small cell lung carcinoma. Clin Lung Cancer 18:207–212. https://doi.org/10.1016/j.cllc.2016.07.009
Buckley AM, Lynam-Lennon N, O’Neill H et al (2020) Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat Rev Gastroenterol Hepatol 17:298–313. https://doi.org/10.1038/s41575-019-0247-2
Jeremic B (2006) Timing of concurrent radiotherapy and chemotherapy in limited-disease small-cell lung cancer: “meta-analysis of meta-analyses.” Int J Radiat Oncol Biol Phys 64:981–982. https://doi.org/10.1016/j.ijrobp.2005.10.034
Withers HR, Taylor JM, Maciejewski B (1988) The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol 27:131–146. https://doi.org/10.3109/02841868809090333
Yom SS (2015) Accelerated repopulation as a cause of radiation treatment failure in non-small cell lung cancer: review of current data and future clinical strategies. Semin Radiat Oncol 25:93–99. https://doi.org/10.1016/j.semradonc.2014.12.002
Davis AJ, Tannock JF (2000) Repopulation of tumour cells between cycles of chemotherapy: a neglected factor. Lancet Oncol 1:86–93. https://doi.org/10.1016/s1470-2045(00)00019-x
Shah MA et al (2020) Treatment of locally advanced esophageal carcinoma: ASCO guideline. J Clin Oncol 38:2677–2694. https://doi.org/10.1200/JCO.20.00866
Wang P et al (2021) A nomogram for the predicting of survival in patients with esophageal squamous cell carcinoma undergoing definitive chemoradiotherapy. Ann Transl Med 9:233. https://doi.org/10.21037/atm-20-1460
Makino T et al (2018) Utility of initial induction chemotherapy with 5-fluorouracil, cisplatin, and docetaxel (DCF) for T4 esophageal cancer: a propensity score-matched analysis. Dis Esophagus. https://doi.org/10.1093/dote/dox130
Acknowledgements
In the protocol design, data collection and analysis, and manuscript writing, we received strong support from clinical and technical colleagues. Their support and helps should be appreciated.
Funding
This work was supported by the National Clinical Key Specialty Construction Program, Fujian provincial Clinical Research Center for Cancer Radiotherapy and Immunotherapy (2020Y2012), and Fujian Science and Technology Innovation Joint Fund Project (2018Y9063).
Author information
Authors and Affiliations
Contributions
JCL, JHL and JJQ designed this study. JJQ, HCL, DMK, YLY, HL, HYZ, LYL, and QHZ contributed to data collection. MQL, JY, MYZ, and ZPW analyzed the data. JCL and JHL supervised the study. JJQ, HCL, YHW, and TXL wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Ethics approval and consent to participate
This study was approved by the ethics committee of Fujian Medical University Cancer Hospital and conducted following the declaration of Helsinki principles and its amendment. All authors read the final manuscript and approved it for publication.
Informed consent
All patients had signed written informed consent before treatment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Qiu, J., Lin, H., Yu, Y. et al. Clinical outcomes and toxicities of locally advanced esophageal squamous cell carcinoma patients treated with early thoracic radiation therapy after induction chemotherapy. Int J Clin Oncol 28, 550–564 (2023). https://doi.org/10.1007/s10147-023-02299-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02299-w