Abstract
Background
Cabozantinib was established as the standard of care for the treatment of patients with renal cell carcinoma (RCC) whose disease had progressed after vascular endothelial growth factor receptor tyrosine kinase inhibitor (VEGFR-TKI) therapy in the global randomized trial METEOR. A phase 2 study was conducted to bridge the findings in METEOR to Japanese patients. Here, we report a biomarker analysis and update the efficacy and safety results of cabozantinib treatment.
Methods
Japanese patients with RCC who received at least one prior VEGFR-TKI were enrolled and received cabozantinib 60 mg orally once daily. The primary endpoint was objective response rate. Secondary endpoints included progression-free survival, overall survival, and safety. Exploratory analyses included the relationship between plasma protein hepatocyte growth factor (HGF) levels and treatment responses.
Results
In total, 35 patients were enrolled. The median treatment duration was 58.3 (range 5.1–131.4) weeks. The objective response rate was 25.7% (90% confidence interval [CI] 14.1–40.6). Kaplan–Meier estimate of median progression-free survival was 11.1 months (95% CI 7.4–18.4). The estimated progression-free survival proportion was 73.1% (95% CI 54.6–85.0) at 6 months. Median overall survival was not reached. Adverse events were consistent with those in METEOR and the safety profile was acceptable. Nonresponders to cabozantinib showed relatively higher HGF levels than responders at baseline.
Conclusions
Updated analyses demonstrate the long-term efficacy and safety of cabozantinib in Japanese patients with advanced RCC after at least one VEGFR-TKI therapy. Responders tended to show lower baseline HGF levels ClinicalTrials.gov Identifier: NCT03339219.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC) is a heterogenous malignancy and the survival of patients with advanced and/or metastatic RCC is very poor [1, 2]. Understanding the biological basis of RCC has led to the development of new targeted agents, including immune checkpoint inhibitors, vascular endothelial growth factor receptor (VEGFR) inhibitors, tyrosine kinase inhibitors (TKIs), and mammalian target of rapamycin (mTOR) inhibitors [3,4,5,6,7,8]. Clear cell (cc) RCC commonly involves mutations in the tumor suppressor Von Hippel–Lindau gene, triggering a decrease in the degradation of hypoxia-inducible factor and an increase in VEGF transcription, which leads to tumor angiogenesis [9]. Thus, inhibitors that target VEGFR, which include sorafenib, sunitinib, pazopanib, axitinib, and lenvatinib, are thought to be effective options with ccRCC patients and in recent years, their combination with IO agents has been established as the standard of care for first-line treatment [10]. However, resistance to VEGF-targeted therapies often arises owing to the upregulation of alternative pro-angiogenic and pro-invasive signaling pathways, including the MET and AXL pathways [11, 12]. Therefore, treatment following prior VEGFR-TKI therapy remains a challenge.
Cabozantinib is a receptor TKI with targets that include MET (c-MET), VEGFR2, RET, AXL, KIT, and TIE-2, which are implicated in tumor growth, metastasis, and angiogenesis [13]. In the phase 3, randomized METEOR trial (ClinicalTrials.gov Identifier: NCT01865747), treatment with cabozantinib improved progression-free survival (PFS), overall survival (OS), and objective response rate (ORR) were compared with treatment with everolimus in patients with metastatic RCC who were treated with at least one prior VEGFR-TKI [14]. Plasma samples from 621 to 658 randomized patients were examined for CA9, hepatocyte growth factor (HGF), MET, growth arrest-specific 6 (GAS6) protein, AXL, VEGF, VEGFR2, and interleukin-8 (IL-8) with the aim of identifying potential prognostic or predictive biomarkers. Most of those candidates were biologically relevant to RCC and included cabozantinib receptor targets and their ligands. In a univariate analysis, low baseline HGF, AXL, and VEGF were prognostic markers for improvements in PFS and OS with cabozantinib [15]. Conversely, a multivariable analysis including International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk groups (favorable, intermediate, or poor) found that low baseline HGF, GAS6, and VEGF were independently prognostic for improved OS with cabozantinib. As a consistent result, low baseline HGF, a key ligand of MET, correlated with improved PFS and OS with cabozantinib treatment. Regardless of the baseline levels of the biomarkers tested, PFS and OS were favorable with cabozantinib versus everolimus [15].
This phase 2 trial (ClinicalTrials.gov Identifier: NCT03339219) was designed to evaluate the efficacy and safety of cabozantinib in Japanese patients with RCC who met similar criteria to those who participated in the METEOR trial [16]. The study met its primary endpoint after short-term follow-up (data cut-off 23 October 2018: ORR, 20.0% (90% confidence interval [CI] 9.8–34.3); clinical benefit rate (CBR), 85.7% (95% CI 69.7–95.2). Cabozantinib has been approved in Japan mainly based on the results from the METEOR study and the Japanese Phase 2 study.
Here, we update our previous report [16] based on the data collected after the initial cut-off date through to the final database lock date (November 19, 2020). Additionally, exploratory analyses included the relationship between plasma levels of HGF, MET, GAS6, and AXL (examined in the METEOR trial) at baseline and treatment responses.
Materials and methods
Study design
This phase 2, open-label, multicenter (19 sites), single-arm trial was conducted to assess the efficacy and safety of cabozantinib in Japanese patients with ccRCC who had received at least one prior VEGFR-TKI therapy [16]. The study protocol and associated documentation were reviewed by institutional review boards at each site. The study was carried out in compliance with the Declaration of Helsinki, the International Council for Harmonisation Good Clinical Practice guidelines, and all applicable local regulations. All patients provided written informed consent before enrollment.
Patients
Patients who met the following inclusion criteria were enrolled in the study: (1) aged ≥ 20 years with a documented histological or cytological diagnosis of ccRCC; (2) measurable disease as per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) and determined by investigators; (3) had received at least one prior VEGFR-TKI therapy (e.g., sorafenib, sunitinib, axitinib, pazopanib, and tivozanib). Further detailed inclusion and exclusion criteria are described in our previous report [16].
Study treatment
Patients received cabozantinib 60 mg once daily orally in the fasted state. Treatment was continued if patients showed clinical benefits or until there were any reasons for treatment discontinuation, including death, adverse events (AEs), clinical deterioration, or a second determination of progressive disease (PD). Dose modifications were permitted for managing AEs with two reduced dose levels (40 mg and 20 mg).
Assessment
Patients were screened within 28 days before the first day of study drug administration (week 1 day 1 [W1D1]). Throughout the study, patient conditions were assessed by the physician and evaluated for efficacy, safety, and health-related quality of life (HRQoL) at scheduled visits. Computed tomography or magnetic resonance imaging of the chest/abdomen/pelvis were performed at screening, 8-week intervals (± 7 days) after W1D1 until the end of the 12-month period, and at 12-week intervals after completion of the first 12 months. Blood and urine samples were obtained every 4 weeks after W1D1.
Questionnaires were collected every 4 weeks after W1D1 until the end of the 9-week period. After week 9, questionnaires were collected every 4 weeks (± 7 days) up to 6 months, and every 8 weeks (± 7 days) until the last tumor assessment.
Endpoints
The primary endpoint was independent review committee (IRC)-assessed ORR, defined as the proportion of patients with complete response (CR) or partial response (PR) as per RECIST 1.1 with a similar observation at least 28 days later. Secondary endpoints included CBR, OS, PFS, and safety evaluation. OS was determined to be the time to death due to any cause occurring after the first dose and PFS was defined as the time from starting treatment to PD as per RECIST 1.1 or death. Safety was assessed based on symptoms and clinical laboratory parameters at a central laboratory. AE severity was graded by the investigators according to Common Terminology Criteria for Adverse Events (CTCAE; Version 4.03). Treatment emergent AEs (TEAEs) were defined as AEs occurring from the first dose to 30 days after receiving the last dose of the study drug.
Other endpoints included HRQoL assessment by the Functional Assessment of Cancer Therapy-Kidney Cancer Symptom Index 19 Item Version (FKSI-19). An exploratory examination of the protein levels of HGF, MET, GAS6, and AXL was performed.
Detection of potential biomarkers
Plasma samples collected on W1D1, week 5 day 1 (W5D1) and week 9 day 1 (W9D1) were tested for potential biomarkers, including HGF, MET, GAS6, and AXL (the protein level at W1D1 was defined as baseline). Detection was achieved using commercially available enzyme-linked immunosorbent assay (ELISA) kits and the following procedures: GAS6 (Human Gas 6 DuoSet ELISA, R&D Systems, Inc., Minneapolis, USA), AXL (Human Axl DuoSet ELISA, R&D Systems, Inc., Minneapolis, USA), HGF (Human HGF Immunoassay, R&D Systems, Inc., Minneapolis, USA), and c-MET (Human c-Met Assay Kit MCM, Immuno-Biological Laboratories Co., Ltd., Gunma, Japan). ELISA kits were validated by using test samples over the range of the standard curve, testing for precision and accuracy, examining diurnally, assessing between-day reproducibility and dilution reproducibility, undertaking freeze–thaw tests and stability tests at room temperature, as well as assessing storage stability for up to 6 months. Validation and measurements were conducted by LSI Medicine Corporation (Tokyo, Japan). Plasma protein concentrations (ng/mL) were obtained by fitting to the standard curve.
Statistical methods
The study aimed to enroll approximately 35 patients, assuming a 10% dropout rate, to provide 32 patients for the analysis of IRC-assessed ORR. The full analysis set (FAS) was used for efficacy analyses, including all patients having received at least one dose of cabozantinib. The safety analysis set used for safety analyses was the same as the FAS. ORR was assessed as point estimates and two-sided 90% exact Clopper–Pearson CIs. The PFS and OS were estimated by Kaplan–Meier analysis. Safety was descriptively summarized. HRQoL values and changes from baseline were descriptively summarized. As an exploratory post hoc analysis, changes from baseline in plasma biomarker levels were evaluated over time at each time point. The differences in baseline plasma biomarker levels between responders and nonresponders were evaluated by two-sample t tests. For descriptive purposes, p < 0.05 was considered statistically significant for each biomarker test result.
Results
Patient disposition
A total of 35 patients with a median age of 63.0 (range 42–84) years were enrolled and all these patients were included in the FAS and the safety analysis set. Of the 35 patients, 15 patients (42.9%) had received immune-oncology (IO) agents. Twenty-four (68.6%) patients had received one prior VEGFR-TKI and eight (22.9%) patients had received two. The most common prior VEGFR-TKI therapies in the total patient group were sunitinib (68.6%) and axitinib (51.4%).
Baseline IMDC risk scores were favorable for 6 (17.1%) patients, intermediate for 22 (62.9%) patients, and poor for 7 (20%) patients [16]. Further detailed baseline characteristics are described in our previous report [16].
Study drug exposure
The median duration of exposure to the study drug was 58.3 weeks (range 5.1–131.4). The median average daily dose was 23.2 mg (range 9.9–60.0 mg) and the corresponding median relative dose intensity was 38.7% (range 16.5–100.0%). All 35 patients discontinued the trial at the point of data cut-off, which included death (n = 1), PD (n = 17), AEs (n = 4), and sponsor termination due to marketing approval of cabozantinib (n = 7) (Fig. 1).
Efficacy assessment
IRC-assessed responses were observed in 9 (25.7%) patients (ORR 25.7%; 90% CI 14.1–40.6), in whom all objective responses were PRs (Table 1). Four (11.4%) patients and 21 (60.0%) patients had progressive disease (PD) and stable disease (SD) as best overall response (BOR), respectively.
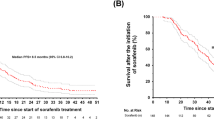
Figure 2 shows a Kaplan–Meier plot of PFS by IRC. A total of 22 PFS events occurred. The follow-up time from enrollment of the last patient through to the last procedure for collection of tumor assessment data was approximately 20 months. The median PFS was 11.1 months (95% CI 7.4–18.4). The proportion of PFS was 73.1% (95% CI 54.6–85.0) at 6 months. The investigator-assessed PFS was similar to that by IRC.
A Kaplan–Meier plot of OS is shown in Fig. 3. A total of 12 deaths occurred during the study and the median OS was not reached. The 1-year and 2-year overall survival rates were 85.4% (95% CI 68.35–93.64%) and 65.7% (95% CI 46.34–79.52%), respectively. The follow-up time from enrollment of the last patient through to the last contact of the last patient was approximately 26 months.
Safety assessment
TEAEs occurred in ≥ 10% of patients are shown in Table 2. All patients experienced at least one AE and a total of 29 patients reported TEAEs of ≥ Grade 3 severity. The dose of study drug was modified (reduced or interrupted) in 33 patients (94.3%) owing to AEs. The most common AEs reported by ≥ 30% of patients were diarrhea, palmar-plantar erythrodysesthesia syndrome, proteinuria, dysgeusia, hypertension, stomatitis, weight decreased, hepatic function abnormal, decreased appetite, and malaise. Most of those symptoms were assessed as drug-related TEAEs. A total of 15 (42.9%) patients experienced one or more serious adverse events (SAEs) and six of whom experienced drug-related SAEs: cholecystitis (n = 2), pneumonia (n = 1), pancreatic enzymes increased (n = 1), hypocalcemia (n = 1), and pneumothorax (n = 1). Study treatment was permanently discontinued in 4 (11.4%) patients owing to AEs that were not related to the disease in the study: proteinuria (n = 2), gastric fistula (n = 1), and pneumothorax (n = 1).
A total of 12 deaths were reported in the safety analysis set. Of these, one death occurred within 30 days after the last dose of the study drug; the death was attributed to disease progression. The other 11 deaths occurred beyond 30 days after the last dose of the study drug and were all attributed to disease progression.
Clinically abnormal serum chemistry parameters detected during treatment are summarized in Table 3.
HRQoL assessment results
Mean changes from baseline in FKSI-19 total scores are shown in Supplementary Fig. 1. The mean FKSI-19 total score at baseline was 59.3 (± 10.17). Over the course of treatment, the mean change from the baseline ranged from − 7.0 to − 0.3. Small increases were observed in the scores of emotional status and functional/well-being status over time. The mean score for treatment side effects decreased at all time points. The mean score for disease-related symptoms decreased at almost all time points (Supplementary Table 1).
Plasma levels of potential biomarkers
Of the 35 patients in the study, post-baseline radiographic tumor assessment was not available for 1 patient and assessment of non-CR/non-PD was made in 2 patients. Additionally, 1 patient with SD with tumor shrinkage was missing baseline biomarker measurements and was omitted from the analysis.
The fold changes from baseline in plasma levels of HGF, MET, GAS6, and AXL at week 5 and week 9 are shown in Table 4. The fold changes of each biomarker (mean) were HGF (1.04, 1.02), MET (1.14, 1.17), GAS6 (1.47, 1.32) and AXL (1.29, 1.35) from baseline to W5D1 or W9D1. MET, GAS6, and AXL levels were significantly increased during treatment (all p < 0.0001). Changes in potential biomarker levels in responders and nonresponders at W5D1 and W9D1 from the baseline are shown in Table 5.
As shown in Fig. 4, 31/33 patients were categorized into two groups by the BOR and the best change in the target lesion sum of diameter (SoD) (Supplementary Fig. 2). Baseline levels of potential biomarkers were shown for each patient group, the responder group (n = 25, CR + PR + SD with tumor shrinkage) or the nonresponder group (n = 6, SD with tumor enlargement + PD). Baseline levels of HGF, MET, and GAS6 tended to be lower in the responder group than the nonresponder group; however, these trends did not reach significance. Conversely, AXL levels tended to be lower in the nonresponders than the responders with no significant difference.
Box plots of potential biomarker levels (n = 31 [responders, n = 25; nonresponders, n = 6]) on W1D1 i.e. baseline. Responders: CR + PR + SD with tumor shrinkage, Nonresponders: SD with tumor enlargement + PD. a HGF; b MET; c GAS6; d AXL. Error bars indicate ± standard deviation (excluding outliers). AXL AXL receptor tyrosine kinase, CR complete response, GAS6 growth arrest-specific 6, HGF hepatocyte growth factor, MET MET receptor tyrosine kinase, PD progressive disease, PR partial response, SD stable disease
Patient distributions from each response group are shown in Table 6. In the nonresponder group, many patients showed higher HGF levels than the median (1114 pg/mL) at baseline. Four out of 5 (80%) nonresponders showed higher HGF levels than 1150 pg/mL, which has been reported to correlate with high-grade tumors and poor survival [17]. MET levels showed similar tendency; all nonresponders indicated higher MET levels than the median (17.64 ng/mL) at baseline.
Discussion
This study was conducted to bridge the results of the cabozantinib arm in the METEOR trial to Japanese patients with a reasonable sample size set for statistical evaluation of IRC-assessed ORR. Support for the long-term efficacy and safety findings for cabozantinib in Japanese patients with ccRCC who had received at least one prior VEGFR-TKI was shown. The IRC-assessed ORR (25.7% [90% CI 14.1–40.6]) and median PFS (11.1 months [95% CI 7.4–18.4]) were numerically higher than those of the cabozantinib arm (n = 330) in the METEOR trial, whereby ORR was 17% (95% CI 13–22) and median PFS (primary endpoint) was 7.4 months (95% CI 6.6–9.1) [14]. TEAEs observed in more than or equal to 10% of patients were evaluated in the METOER trial and this study (Table 2). Diarrhea, hypertension and palmar-plantar erythrodysesthesia syndrome were observed as the major adverse events under cabozantinib treatment in both studies. The other safety profiles were also similar between the studies. Overall, safety was considered to be manageable [14].
Almost half of the enrolled patients (15/35, 42.9%) had received IO agents with or without a TKI before starting the study protocol. IO agents have been evolving the treatment of cancer worldwide since the approval of the ipilimumab in 2011 [18]. IO agents are now recommended as the first-line treatment option for patients with RCC in many countries, including Japan [19, 20]. There is an exploratory result from the pooled analysis of the cabozantinib arm of the METEOR trial and this Japanese phase 2 study, in which ORR was 21.2% (95% CI 9.0–38.9%) and 17.2% (95% CI 13.3–21.7%) in patients with prior-IO and no prior-IO, respectively [21]. Interest in using subsequent therapies after IO agents and combination therapies using IO agents has been raised. In this study, no stratified analysis with treatment sequences has been conducted, therefore, no additional information was obtained.
Predictive biomarkers associated with cabozantinib treatment responses will support the identification of patients who may benefit from treatment. Currently, no plasma biomarkers that are consistently predictive or prognostic for an improved benefit with cabozantinib treatment have been found. For example, on-treatment changes in HGF appeared prognostic for improved PFS or OS with cabozantinib in univariate analyses, but not independently prognostic in multivariate analyses in the METEOR trial [15].
In our study described here, an exploratory examination of four potential biomarkers suggested in the METEOR trial was undertaken using ELISA and plasma samples, which is a feasible method in clinical practice. Plasma levels of MET and AXL, which are membrane receptors, were assessed as surrogate markers for these receptors. Fold changes from baseline in the median/mean at week 5 were similar to the results in the cabozantinib arm of the METEOR trial [15]. In addition, moderate elevations of MET, GAS6, and AXL were observed at week 5 and were still higher than the baseline levels at week 9 (Table 4). The clinical meaning of these elevations remains unclear. HGF levels were stable during this period. We assessed the on-treatment changes of these factors for responders and nonresponders; however, minimal difference was observed between groups (Table 5). As an additional consideration, we used different definition for responders and nonresponders (Supplementary Fig. 3, Supplementary Table 2 and 3). Responders were composed of CR and PR, PD and SD were included in nonresponders. With this categorization, HGF was significantly lower in responders. HGF might be a predictive biomarker of cabozantinib treatment. All the other factors showed similar trends with those when SD with tumor shrinkage was included in responders.
Tanimoto et al., previously reported that serum HGF levels were significantly higher in patients with ccRCC (1070.7 pg/mL, n = 45) than healthy patients (728 pg/mL, n = 45), and HGF > 1150 pg/mL correlated with poor survival [17]. In the METEOR trial, PFS and OS were longer with cabozantinib versus everolimus in both patient populations with low or high baseline HGF, suggesting a benefit with cabozantinib treatment irrespective of baseline HGF levels [15]. In this study, we evaluated tumor shrinkage as the indicator of cabozantinib treatment response and the correlation of baseline HGF levels (Fig. 4). Responders tended to show lower baseline HGF levels, which was similar to the results from METEOR.
In summary, support for the long-term efficacy and safety findings for cabozantinib in Japanese patients with ccRCC who had received at least one prior VEGFR-TKI was shown in our study. Responders tended to show lower baseline HGF levels.
Data availability
The data sets, including the redacted study protocol, redacted statistical analysis plan, and individual participant data supporting the results reported in this article, will be made available within 3 months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
References
Inamura K (2017) Renal cell tumors: understanding their molecular pathological epidemiology and the 2016 WHO classification. Int J Mol Sci 18(10):2195
Padala SA, Barsouk A, Thandra KC et al (2020) Epidemiology of renal cell carcinoma. World J Oncol 11(3):79–87
Barry RE, Krek W (2004) The von Hippel-Lindau tumour suppressor: a multi-faceted inhibitor of tumourigenesis. Trends Mol Med 10(9):466–472
Jonasch E, Walker CL, Rathmell WK (2021) Clear cell renal cell carcinoma ontogeny and mechanisms of lethality. Nat Rev Nephrol 17(4):245–261
Kaelin WG (2008) The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 8(11):865–873
Rini BI, Atkins MB (2009) Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol 10(10):992–1000
Rankin EB, Fuh KC, Castellini L et al (2014) Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci USA 111(37):13373–13378
Choueiri TK, Motzer RJ (2017) Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 376(4):354–366
Na X, Wu G, Ryan CK et al (2003) Overproduction of vascular endothelial growth factor related to von Hippel-Lindau tumor suppressor gene mutations and hypoxia-inducible factor-1 alpha expression in renal cell carcinomas. J Urol 170(2 Pt 1):588–592
Motzer R, Alekseev B, Rha SY et al (2021) Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 384(14):1289–1300
Bergers G, Hanahan D (2008) Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 8(8):592–603
Qu L, Ding J, Chen C (2016) Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell 29(5):653–668
Yakes FM, Chen J, Tan J et al (2011) Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 10(12):2298–2308
Choueiri TK, Escudier B, Powles T et al (2016) Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 17(7):917–927
Powles T, Choueiri TK, Motzer RJ (2021) Outcomes based on plasma biomarkers in METEOR, a randomized phase 3 trial of cabozantinib vs everolimus in advanced renal cell carcinoma. BMC Cancer 21(1):904
Tomita Y, Tatsugami K, Nakaigawa N et al (2020) Cabozantinib in advanced renal cell carcinoma: A phase II, open-label, single-arm study of Japanese patients. Int J Urol 27:952–959
Tanimoto S, Fukumori T, El-Moula G et al (2008) Prognostic significance of serum hepatocyte growth factor in clear cell renal cell carcinoma: comparison with serum vascular endothelial growth factor. J Med Invest 55(1–2):106–111
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723
Japanese guideline: Clinical Practice Guideline for Renal Cancer 2017 (2019 updated). Available from URL: https://www.urol.or.jp/lib/files/other/guideline/33_renal_cancer_2017_rev2020_info.pdf. Accessed Jun 2020
National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Kidney Cancer version 1.2023. Available from URL: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. Accessed 17 Jun 2022
Oya M, Tamada S, Tatsugami K et al (2020) ASCO Annual Meeting Abstract 5089
Acknowledgements
The authors would like to thank all patients and their families and caregivers, the Cabozantinib-2001 investigators and their team members at each study site and colleagues from Takeda Pharmaceutical Company Limited. Graphical assistance was provided by MIMS Japan Co., Ltd., Tokyo, Japan, and editorial support was provided by Steven Inglis, PhD, and Angelica Papanicolaou, PhD, of Oxford PharmaGenesis, Melbourne, Australia, which was funded by Takeda Pharmaceutical Company Limited, Tokyo, Japan, in accordance with Good Publication Practice 3 (GPP3). Kazumi Nomura, PhD (Takeda Pharmaceutical Company Limited) is acknowledged for medical writing assistance.
Funding
This study was sponsored by Takeda Pharmaceutical Company Limited.
Author information
Authors and Affiliations
Contributions
AK and SK were involved in the study design. Study investigators included NN, YT, ST, KT, TO, MO, HK, YM, NS, KN, MN, NM and YM. Patients were enrolled by NN, YT, ST, KT, TO, MO, HK, YM, NS, KN, MN, NM and YM. AK and SK contributed to data collection, assembly, and analysis. All authors were involved in the interpretation of data, and revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Noboru Nakaigawa: Honoraria from Biopharma, BMS, Merck, MSD, Ono Pharmaceutical, Pfizer and Takeda. Yoshihiko Tomita: Research grants from Astellas, Ono Pharmaceutical and Takeda; and honoraria from Astellas, BMS, Ono Pharmaceutical and Pfizer. Satoshi Tamada: Honoraria from Merck Biopharma, MSD, Pfizer and Takeda. Katsunori Tatsugami: Honoraria from BMS, Merck Biopharma, MSD, Ono Pharmaceutical, Pfizer and Takeda. Takahiro Osawa: Honoraria from Takeda. Mototsugu Oya: Honoraria from Bayer, Bristol-Myers Squibb, Eisai, Merck, MSD, Novartis, Ono, Pfizer, and Takeda; scholarship donations from Bayer, Ono Pharmaceutical and Takeda. Hiroomi Kanayama: No conflict of interest to disclose. Yuji Miura: Research funding from MSD, Ono Pharmaceutical; and honoraria from Bristol-Myers Squibb, Eisai, and Takeda. Naoto Sassa: No conflict of interest to disclose. Kazuo Nishimura: Honoraria from Merck Biopharma. Masahiro Nozawa: Honoraria from Takeda. Naoya Masumori: Research funding from Astellas, Bristol Myers Squibb and MSD; honoraria from Astellas, Astrazeneca, Bayer, Janssen, Kissei and Takeda. Yasuhide Miyoshi: No conflict of interest to disclose. Shingo Kuroda: An employee and stock owner of Takeda Pharmaceutical Company Limited. Akiko Kimura: An employee of Takeda Pharmaceutical Company Limited.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Nakaigawa, N., Tomita, Y., Tamada, S. et al. Final efficacy and safety results and biomarker analysis of a phase 2 study of cabozantinib in Japanese patients with advanced renal cell carcinoma. Int J Clin Oncol 28, 416–426 (2023). https://doi.org/10.1007/s10147-022-02283-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02283-w