Abstract
Aims
To determine the incidence, clinical presentation, and outcome of methotrexate (MTX) associated neurotoxicity in pediatric patients treated for osteosarcoma, with the aim of identifying possible risk factors and suggesting recommended treatment for these sequelae.
Materials and methods
All medical files of patients treated for osteosarcoma in a single pediatric haemato-oncology center between November 2011 and August 2021 were retrospectively reviewed. All patients were treated according to the EURAMOS AOST0331 protocol, using cisplatin, doxorubicin, and high-dose MTX at a dose of 12 g/m2 over 4 h.
Results
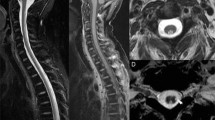
Seventy-eight patients with osteosarcoma were identified (age range 5 to 23 years, 42 males). Seven patients (9%) sustained neurotoxicity following treatment with high-dose MTX. Manifestations of neurotoxicity included among others, generalized seizures, confusion, encephalopathy, dysarthria, and choreiform movements. All but one episode occurred following two sequential cycles of high-dose MTX. All 7 had subacute toxicity, 5–10 days following MTX administration, and 1 had both acute and subacute toxicity. Brain MRI was performed for all patients and demonstrated typical MRI changes attributed to MTX neurotoxicity in 4 of them. Two patients received aminophylline; one patient received dextromethorphan. Patients with normal MRI imaging resumed MTX therapy without any sequels. No risk factors were found for high-dose MTX-related toxicity occurrence.
Conclusions
The time of risk of neurotoxicity due to high-dose MTX treatment for osteosarcoma is days 5–10 following two sequential treatment cycles. These findings together with treatment options for these adverse effects should be detailed in the therapeutic protocol of MTX use among pediatric patients with osteosarcoma.

Similar content being viewed by others
References
Mirabello L, Troisi RJ, Savage SA (2009) Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer 115:1531–1543
Isakoff MS, Bielack SS, Meltzer P et al (2015) Osteosarcoma: current treatment a collaborative pathway to success. J Clin Oncol 33:3029–3035
Goldman ID, Matherly LH (1985) The cellular pharmacology of methotrexate. Pharmacol Ther 28:77–102
Allegra CJ, Fine RL, Drake JC et al (1986) Effect of methotrexate on intracellular folate pools in human MCF breast cancer cells. J Biol Chem 261:6478–6485
Inaba H, Khan RB, Laningham FH et al (2008) Clinical and radiological characteristics of methotrexate induced acute encephalopathy in pediatric patients with cancer. Ann Oncol 19:178–184
Asato R, Akiyama Y, Ito M et al (1992) Nuclear magnetic resonance abnormalities of the cerebral white matter in children with acute lymphoblastic leukemia and malignant lymphoma during and after central nervous system prophylactic treatment with intrathecal methotrexate. Cancer 70:1997–2004
Howard SC, McCormick J, Pui CH et al (2016) Preventing and managing toxicities of high-dose methotrexate. Oncologist 21:1471–1482
Stary J, Zimmermann M, Campbell M et al (2014) Intensive chemotherapy for childhood acute lymphoblastic leukemia: results of the randomized intercontinental trial ALL IC-BFM 2002. J Clin Oncol 32:174–184
Bhojwani D, Sabin ND, Pei D et al (2014) Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol 32:949–959
Allen JC, Rosen G, Mehta BM et al (1980) Leukoencephalopathy following high-dose iv methotrexate chemotherapy with leucovorin rescue. Cancer Treat Rep 64(12):1261–1273
Mittal R, Mottl H, Nemec J (2005) Acute transient cerebral toxicity associated with administration of high-dose methotrexate. Med Princ Pract. 14(3):202–204. https://doi.org/10.1159/000084641
Lahi Z, Janardhan S, Dave M et al (2022) Ketamine as an adjunct for treatment of methotrexate-induced neurotoxicity. J Pediatr Hematol Oncol. 44(2):e512–e513. https://doi.org/10.1097/MPH.0000000000002366
Terasawa Y, Nakane S, Ohnishi T et al (2007) Case of methotrexate encephalopathy: findings on diffusion tensor image and correlation with clinical outcome. Rinsho Shinkeigaku 47(2–3):79–84
Ayalon I, Friedman S, Binenbaum Y et al (2019) A case of methotrexate neurotoxicity presented as status epilepticus, encephalopathy, and high fever. J Investig Med High Impact Case Rep 7:2324709619862311. https://doi.org/10.1177/2324709619862311
Müller J, Kralovánszky J, Adleff V et al (2008) Toxic encephalopathy and delayed MTX clearance after high-dose methotrexate therapy in a child homozygous for the MTHFR C677T polymorphism. Anticancer Res 28(5B):3051–3054
Mittal R, Mottl H, Nemec J (2005) Acute transient cerebral toxicity associated with administration of high-dose methotrexate. Med Princ Pract 14(3):202–204. https://doi.org/10.1159/000084641
Marina NM, Smeland S, Bielack SS et al (2016) Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised ontrolled trial. Lancet Oncol 17:1396–1408
Taylor ZL, Mizuno T, Punt NC et al (2020) MTXPK.org: a clinical decision support tool evaluating high-dose methotrexate pharmacokinetics to inform post-infusion care and use of glucarpidase. Clin Pharmacol Ther. 108(3):635–643. https://doi.org/10.1002/cpt.1957
Mateos MK, Marshall GM, Barbaro PM et al (2021) Methotrexate-related central neurotoxicity: clinical characteristics, risk factors and genome-wide association study in children treated for acute lymphoblastic leukemia. Haematologica. https://doi.org/10.3324/haematol.2020.268565
Bernini JC, Fort DW, Griener JC et al (1995) Aminophylline for methotrexate-induced neurotoxicity. Lancet 345:544–547
Jaksic W, Veljkovic D, Pozza C et al (2004) Methotrexate-induced leukoencephalopathy reversed by aminophylline and high-dose folinic acid. Acta Haematol 111(4):230–232. https://doi.org/10.1159/000077573
Ganesan P, Bajpai P, Shah A et al (2014) Methotrexate induced acute encephalopathy-occurrence on re-challenge and response to aminophylline. Indian J Hematol Blood Transfus 30:105–107. https://doi.org/10.1007/s12288-013-0275-y
Drachtman RA, Cole PD, Golden CB et al (2002) Dextromethorphan is effective in the treatment of subacute methotrexate neurotoxicity. Pediatr Hematol Oncol 19:319–327
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Peled, Y., Levin, D., Shiran, S. et al. Prevalence and management of methotrexate-induced neurotoxicity in pediatric patients with osteosarcoma: a single-center experience. Int J Clin Oncol 27, 1372–1378 (2022). https://doi.org/10.1007/s10147-022-02184-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02184-y