Abstract
Background
Based on results from Japanese post-marketing surveillance, exploratory analyses were performed to investigate real-world outcomes of radium-223 for metastatic CRPC (mCRPC) according to patient characteristics.
Methods
This non-interventional, prospective study enrolled mCRPC patients selected for radium-223 treatment in clinical practice. Six-month safety and effectiveness were evaluated in subgroups who had/had not received prior chemotherapy (prior-chemo/no prior-chemo groups), and a subgroup who had not received concomitant androgen-receptor axis-targeted agents (ARATs).
Results
In the overall population (n = 296), the prior-chemo group (n = 126) tended to have more bone metastases, more analgesic use, and higher prostate-specific antigen values than the no prior-chemo group (n = 170). Incidences of treatment-emergent adverse events (TEAEs), drug-related TEAEs, and ≥ grade 3 drug-related hematological TEAEs were 47% vs. 53%, 25% vs. 29%, and 4% vs. 7% in the no prior-chemo and prior-chemo groups, respectively. Incidences of TEAEs (61%), drug-related TEAEs (36%), and ≥ grade 3 drug-related hematological events (12%) were numerically higher in 33 patients who had received two lines of prior chemotherapy. Multivariate analysis showed that two lines of prior chemotherapy, and hemoglobin, platelet, and lactate dehydrogenase values were baseline factors significantly related to ≥ grade 2 platelet count decreased. Safety and effectiveness in patients without concomitant ARATs (n = 201) were similar to those in the overall population.
Conclusion
In a real-life setting, radium-223 was well tolerated irrespective of prior chemotherapy, but relatively higher incidences of TEAEs and hematotoxicities were suggested in patients with two lines of prior chemotherapy, possibly reflecting more advanced disease. Radium-223 safety and effectiveness in patients without concomitant ARATs were favorable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As prostate cancer (PC) advances, bone metastasis is common, ultimately occurring in 80–90% of patients [1, 2]. Bone metastasis is associated with reduced quality of life (QoL), and with increased disability, risk of death, and treatment costs [2,3,4,5,6,7,8,9,10,11]. Therefore, it is essential to manage bone metastasis effectively as part of metastatic castration-resistant prostate cancer (mCRPC) treatment.
Radium-223 (Ra-223) is the first targeted alpha therapy that prolongs overall survival (OS) in patients with bone mCRPC [12]. In a randomized phase III study in patients with CRPC and symptomatic bone metastases (ALSYMPCA), Ra-223 significantly prolonged OS compared with placebo (median: 14.9 versus 11.3 months; hazard ratio: 0.70, p < 0.001). Ra-223 also significantly prolonged time to first symptomatic skeletal event and improved QoL compared with placebo [12].
Ra-223 was approved in Japan for mCRPC treatment in March 2016 and, to date, has been used in several thousand patients. Because understanding of the safety and usefulness of an approved agent in clinical settings is essential, post-marketing surveillance (PMS) has been conducted in Japan since June 2016, in accordance with Japanese regulations. Primary PMS study results, the safety and effectiveness of Ra-223 in the total study population (n = 296), have already been reported, with no new safety concerns identified during the 6-month observation period [13].
The characteristics of patients with CRPC are heterogeneous and, thus, to select the most appropriate treatment, it is necessary to consider the treatment history and characteristics of individual patients. Regarding safety, chemotherapy is generally known to be associated with bone marrow toxicity [14, 15], and hematological treatment-emergent adverse events (TEAEs) are common with Ra-223 treatment [12]. Therefore, the safety and usefulness of Ra-223 based on the status of previous chemotherapy use is of specific interest. Indeed, prior chemotherapy use in non-Japanese patients was shown to lead to an increased risk of hematologic toxicity under Ra-223 treatment in ALSYMPCA [16, 17] and observational [18] studies. This trend is thought to be consistent in Japanese patients, but corroborative data from Japanese patients are lacking. Additionally, we have to consider that patient characteristics often differ due to differences in healthcare systems, reimbursement status, and recommended therapeutic strategies among countries.
It is also essential to assess agents in the context of current real-world treatment strategies. Clinical trials, such as ALSYMPCA and a Japanese phase II study [19], did not include patients who had received prior or concomitant treatment with second-generation androgen-receptor axis-targeted agents (ARATs). Because, nowadays, ARATs are used commonly for PC treatment, it is important to reassess the real-world outcomes of Ra-223 in patients who are treated sequentially with various agents. In particular, data in patients without concomitant use of ARATs are meaningful for evaluating the effects of Ra-223 itself.
In order to obtain further information about the optimal patient characteristics for Ra-223 treatment, and the safety and effectiveness of Ra-223 itself, we performed exploratory subgroup analyses of data from the Japanese PMS study in patients with and without prior chemotherapy, and in patients who did not receive concomitant second-generation ARATs. Also, because the occurrence of hematotoxicity is a safety interest, we investigated patient baseline characteristics contributing to the occurrence of hematotoxicity with Ra-223 treatment.
Patients and methods
Study design and patients
This was a non-interventional, prospective, multicenter, single-cohort PMS study performed in Japan (ClinicalTrials.gov NCT02803437). Patients with CRPC and bone metastasis, who were selected for treatment with Ra-223 according to the participating physicians’ routine clinical practice, were eligible for inclusion. The target sample size was 300 patients, and the enrollment period was set at 18 months. The observation period for the primary study was 6 months, from the first administration of Ra-223 to 30 days after the last administration. Ra-223 was administered at a dose of 55 kBq/kg every 4 weeks for up to six cycles.
The institutional review board at each participating center approved the study, which was conducted in accordance with the Declaration of Helsinki and Japanese regulations on Good Post-marketing Study Practice. According to Japanese regulations, written informed consent from patients was not required.
Endpoints
The primary endpoints were TEAEs and drug-related TEAEs. TEAEs were defined as events which occurred following the first administration of Ra-223 until 30 days after the last administration. An important focus was hematological TEAEs, including anemia, leukopenia, neutropenia, thrombocytopenia, pancytopenia, and other hematological events. The occurrence of skeletal-related events (SREs) was also assessed; SREs included pathological bone fractures, spinal cord compression, events requiring external-beam radiotherapy (EBRT) to relieve skeletal symptoms, events requiring orthopedic surgical intervention, and hypercalcemia. Secondary endpoints included laboratory findings, such as total alkaline phosphatase (t-ALP) and prostate-specific antigen (PSA) levels from baseline.
Statistical analysis
Statistical analyses were generally exploratory and descriptive in nature. For the analysis based on chemotherapy history, patients were divided into those who had received chemotherapy (docetaxel, cabazitaxel, and other chemotherapy) before the first administration of Ra-223 (defined as prior-chemo group) and those who had not (defined as no prior-chemo group). For the prior-chemo group, results are also shown for the subgroup of patients who had received two lines of prior chemotherapy (defined as two lines of prior-chemo group). In the subgroup analysis of patients who received Ra-223 without concomitant ARATs, only patients with treatment periods for enzalutamide or abiraterone that did not overlap with those for Ra-223 were included (defined as without concomitant ARATs group). Because one patient had received docetaxel overlapping with the Ra-223 treatment period, the patient was excluded from the without concomitant ARATs group. Also, in this manuscript, "(second-generation) ARAT" always refers to abiraterone or enzalutamide. Univariate and multivariate analysis with logistic regression analysis (forward–backward stepwise selection method, significance level of 0.05) for the occurrence of hematotoxicity was performed using baseline patient characteristics and prior treatments as individual risk factors. In this analysis, hematotoxicity was defined as the occurrence of hematological laboratory abnormalities (decline) of ≥ CTCAE grade 2; patients with grade 3/4 abnormalities were too few for meaningful analysis. In addition, to investigate the risk factors for hematotoxicity objectively, hematological laboratory abnormalities, not TEAEs, were used. Corresponding baseline hematological laboratory values were always added into the model of multivariate analysis regardless of the univariate analysis results (e.g. a variable of baseline hemoglobin was always added into the model for investigating the occurrence of decreased hemoglobin), given that these factors were expected to correlate with respective hematotoxicities. Log transformation was performed for baseline variables with heavily-skewed distributions (e.g., t-ALP and PSA). Other statistical analysis details are described in Supplementary Methods.
Results
Baseline characteristics and prior and concomitant therapies
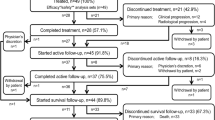
Overall, 334 patients were enrolled into the PMS study between June 2016 and November 2017. In November 2019, data for 299 patients were available, and 296 patients were included in the safety and effectiveness analysis sets; three patients who did not receive Ra-223 were excluded. The median observation period was 5.6 (range 1.0–10.6) months.
Baseline characteristics and prior/concomitant therapies are presented in Table 1 according to prior chemotherapy status. Among the 296 patients, 170 (57%) were in the no prior-chemo group, while 126 (43%) were in the prior-chemo group and, of these, 33 (26% of the prior-chemo group, 11% of the overall population) had received two lines of chemotherapy.
The proportion of patients with WHO’s cancer pain ladder score of 0 was numerically smaller (60% vs 75%) and the median PSA value (33.8 vs 17.3 ng/mL) was higher in the prior-chemo group than the no prior-chemo group. The prior-chemo group tended to have more bone metastases; the proportions of patients with extent of disease (EOD) 1 were 21% and 34%, and, for EOD3, were 37% and 29% in the prior-chemo and no prior-chemo groups, respectively. The prior-chemo group was more likely to have previously received ARATs (78% vs 65%) and EBRT (26% vs 14%).
The two lines of prior-chemo group tended to have more advanced disease, indicated by a smaller proportion having PS 0 and WHO’s cancer pain ladder 0, and a higher median PSA value, even compared with the overall prior-chemo group.
Among the overall population (n = 296), 201 (68%) patients were treated with Ra-223 without receiving concomitant ARATs. Overall, the baseline characteristics and pattern of prior or concomitant therapies in the without concomitant ARATs group were similar to those of the overall population (Supplementary Table 1).
Ra-223 exposure
In the overall population, 69% of patients completed six cycles of Ra-223 treatment, and 75% completed five or six cycles. More patients in the no prior-chemo group completed six cycles of Ra-223 (75%) compared to the prior-chemo group (60%). Patients in the two lines of prior-chemo group were notably less likely to complete six cycles of Ra-223 (39%). In the without concomitant ARATs group, 65% of patients completed six cycles of Ra-223.
Safety
TEAEs and drug-related TEAEs
Among the overall population, TEAEs and drug-related TEAEs occurred in 49% and 26% of patients, respectively; the majority of events were grade 1 or 2 in severity.
TEAEs and drug-related TEAEs in the no prior-chemo group and prior-chemo group are summarized in Table 2. The incidences of TEAEs and drug-related TEAEs were 47% vs 53% and 25% vs 29% in the no prior-chemo group and prior-chemo group, and for ≥ grade 3 TEAEs/drug-related TEAEs were 15% vs 23% and 5% vs 9%, respectively. The incidences of these events tended to be even higher in the two lines of prior-chemo group.
Among drug-related hematological TEAEs, the most common event was anemia (13% of the overall population) (Supplementary Table 2); this trend was similar across groups (Table 2). The incidence of hematological drug-related TEAEs of any grade was similar in the no prior-chemo group and the prior-chemo group (17% vs 18%), and was numerically higher in the two lines of prior-chemo group (24%). Among hematological drug-related TEAEs, the incidences of serious events were 1%, 5%, and 6%, and for events ≥ grade 3 were 4%, 7%, and 12% in the no prior-chemo, prior-chemo, and two lines of prior-chemo groups, respectively.
The incidences of TEAEs and drug-related TEAEs in the without concomitant ARATs group were similar to those for the overall population (Supplementary Table 2).
Hematological laboratory abnormalities
Hematological laboratory abnormalities that occurred during Ra-223 treatment are summarized in Table 3. The incidence of ≥ grade 3 hemoglobin decreased was numerically higher in the prior-chemo group (14%) compared with the no prior-chemo group (7%), tending to be even higher in the two lines of prior-chemo group (25%). This trend was also similar for ≥ grade 3 platelet count decreased; the incidences were 1%, 5%, and 13% in the no prior-chemo, prior-chemo, and two lines of prior-chemo groups, respectively. The incidences of ≥ grade 3 neutrophil count decreased were low across all groups (from 0 to 2%).
The overall incidence of hematological laboratory abnormalities in the without concomitant ARATs group was similar to that in the overall population.
Related baseline characteristics for ≥ grade 2 hematological laboratory abnormalities
Patient baseline characteristics related to the occurrence of ≥ grade 2 hematological abnormalities are shown in Table 4. Multivariate analysis showed that, low hemoglobin high PSA, and high t-ALP at baseline were significantly related to the occurrence of ≥ grade 2 hemoglobin decrease. With respect to the occurrence of ≥ grade 2 platelet count decrease, low hemoglobin, low platelet count, high LDH, and two lines of prior chemotherapy were significantly related. Regarding the occurrence of decreased neutrophil count, because the availability of the model was low (0.635) due to missing data, this result was considered as a reference only.
Skeletal-related events and fractures
A drug-related SRE occurred in one patient in the overall population (0.3%); the patient was in the prior-chemo group (0.8%) and also belonged to the without concomitant ARATs group (0.5%). The SRE was an event requiring EBRT.
During the observation period, a total of five fractures were reported, all of which were judged unrelated to Ra-223 by investigators; fractures included femoral (n = 2), rib (n = 1), femoral neck (n = 1), and pathological bone (n = 1). Only one of the five patients had received concomitant bone-modifying agents.
Effectiveness
Percent changes in t-ALP and PSA over 24 weeks
Percent changes from baseline in t-ALP and PSA over 24 weeks are summarized in Fig. 1, Supplementary Fig. 1, and Supplementary Table 3.
t-ALP decreased in most patients across all groups during the observation period and the transition of changes was not evidently different across groups. t-ALP reductions were observed from week 4 after the first administration of Ra-223 and were sustained throughout the observation period.
Median percent changes in PSA from baseline were shown to increase in all groups, although the increases were relatively smaller in the no prior-chemo group compared to those in the prior-chemo group; the increases tended to be larger in patients who had two lines of prior chemotherapy. The transition of changes in PSA over 24 weeks in the without concomitant ARATs group was similar to that for the overall population.
t-ALP and PSA changes at Week 12 by category
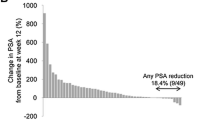
When categorized by extent of change at week 12, any decline in t-ALP was observed in 63–82% of patients across groups (Supplementary Table 3).
At week 12, any decline in PSA was observed in 34%, 23%, and 12% of patients in the no prior-chemo, prior-chemo, and two lines of prior-chemo groups, respectively. The proportions of patients with any decline in PSA at week 12 were similar in the without concomitant ARATs group and overall population.
Discussion
This PMS study is the largest prospective observational study of Ra-223 conducted in Japan. The primary results for the overall population (n = 296) have been published previously [13]. Considering that Ra-223 has been used for patients with various backgrounds in Japanese clinical settings, we performed exploratory analyses to investigate its safety and effectiveness in subgroups by status of prior chemotherapy or concurrent use of ARATs.
In this study, 43% of patients had received chemotherapy before Ra-223 treatment. In the prior-chemo group, some baseline patient characteristics suggesting more advanced PC were observed compared to the no prior-chemo group, including high PSA values and more bone metastases. Additionally, other treatments, including ARATs and EBRT, were used more frequently before Ra-223 in the prior-chemo group, indicating that this population was heavily treated. Overall, Ra-223 was well tolerated in all groups. However, the incidences of TEAEs/drug-related TEAEs with serious or ≥ grade 3 events, and ≥ grade 3 hematological TEAEs, were numerically higher in the prior-chemo group than the no prior-chemo group. Notably, baseline characteristics aligned with advanced PC and a more frequent incidence of TEAEs were evident in the two lines of prior-chemo group, suggesting that more adverse events were presumably due to more advanced disease stage in these patients.
In multivariate analysis, the selected significant factors for hematological abnormalities were low hematological laboratory values at baseline or baseline characteristics suggesting advanced primary disease. While some selected factors were different from those in ALSYMPCA [17], the trend was not contradictory. It is important to understand that differences in era, patient population, and study design may affect the results; for example, cabazitaxel had not yet been approved at initiation of ALSYMPCA. A history of docetaxel use was a significant factor for thrombocytopenia in ALSYMPCA [17], and prior treatment with two lines of chemotherapy was a selected factor for decreased platelet count in the current study. In line with the fact that the incidence of overall TEAEs was especially higher in the two lines of prior-chemo group compared to other groups, this highlights the importance of monitoring patients and paying attention to the occurrence of adverse events particularly in Japanese patients with a history of intensive chemotherapy.
While the decline in t-ALP from baseline was observed in many patients across all groups, the median of percent change in PSA during the Ra-223 treatment period was relatively higher in the prior-chemo group. Notably, the proportion of patients who completed six cycles of Ra-223 was smaller in the prior-chemo group, especially in the two lines of prior-chemo group. Given that some patients might discontinue Ra-223 when PSA increases are unbearable, PSA dynamics might have influenced, partially at least, the Ra-223 completion rate. Unfortunately, because detailed information about disease progression, as a reason for discontinuation, was not obtained in this study, the profile of disease progression was unclear. However, as recommended by guidelines [20], the assessment of treatment effect and decisions to change treatment should be performed comprehensively.
Consensus about the best CRPC treatment sequence has not yet been reached [21, 22], and the best sequence with Ra-223 and other agents also remains unclear. From the sub-analysis of ALSYMPCA, it was shown that, compared with placebo, the Ra-223 survival benefit was sustained regardless of prior chemotherapy [16], and that Ra-223 treatment did not affect subsequent chemotherapy [23]. Also, considering these reports, current subgroup results indicating tendencies of fewer TEAEs and a higher Ra-223 completion rate in the no prior-chemo group might partially suggest that Ra-223 treatment before chemotherapy may be beneficial to patients; however, to reach decisive conclusions, findings regarding the effect of each treatment sequence on overall survival are awaited.
We also investigated the safety and effectiveness of Ra-223 in patients without concomitant use of second-generation ARATs. The fact that most outcomes in this subgroup were similar to those in the overall population provides encouragement that the safety and effectiveness of Ra-223 itself in current real-world settings is sustained without concomitant life-prolonging CRPC treatment, and in patients receiving various sequential CRPC treatments.
There are some limitations to the results, including small sample size and single-cohort study design. Subgroup analysis results from a single cohort should be interpreted cautiously, because they are often attributed to differences in baseline characteristics. However, our analysis reflects real-world patients and should help to develop treatment strategies in clinical practice without influence from patient selection or interventions. Also, although the effect of treatment on QoL is an important aspect for patients, endpoints related to QoL were not included in the protocol of this study because PMS is a survey to mainly investigate the drug’s safety. Prospective investigations about QoL, as well as quality adjusted life-year, in Japanese patients treated with Ra-223 would be expected in the future. Follow-up for this study, up to 3 years, is ongoing and future findings, including prognosis and fracture information, will further strengthen the findings from this exploratory analysis.
Change history
09 March 2021
Electronic Supplementary Material is included.
References
Pezaro C, Omlin A, Lorente D et al (2014) Visceral disease in castration-resistant prostate cancer. Eur Urol 65(2):270–273
Kirby M, Hirst C, Crawford ED (2011) Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract 65(11):1180–1192
Broder MS, Gutierrez B, Cherepanov D et al (2015) Burden of skeletal-related events in prostate cancer: unmet need in pain improvement. Support Care Cancer 23(1):237–247
Yong C, Onukwugha E, Mullins CD (2014) Clinical and economic burden of bone metastasis and skeletal-related events in prostate cancer. Curr Opin Oncol 26(3):274–283
McDougall JA, Bansal A, Goulart BH et al (2016) The clinical and economic impacts of skeletal-related events among medicare enrollees with prostate cancer metastatic to bone. Oncologist 21(3):320–326
Weinfurt KP, Li Y, Castel LD et al (2005) The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol 16(4):579–584
Kurth H, McKiernan J, Bentkover JD et al (2005) Quality of life and pain among prostate cancer patients with bone metastases. J Clin Oncol 23(suppl 16):4747
Sathiakumar N, Delzell E, Morrisey MA et al (2011) Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis 14(2):177–183
Howard LE, De Hoedt AM, Aronson WJ et al (2016) Do skeletal-related events predict overall survival in men with metastatic castration-resistant prostate cancer? Prostate Cancer Prostatic Dis 19(4):380–384
Hagiwara M, Delea TE, Saville MW et al (2013) Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis 16(1):23–27
Jayasekera J, Onukwugha E, Bikov K et al (2014) The economic burden of skeletal-related events among elderly men with metastatic prostate cancer. Pharmacoeconomics 32(2):173–191
Parker C, Nilsson S, Heinrich D et al (2013) Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369(3):213–223
Takahashi S, Kakehi Y, Masumori N et al (2020) Safety and effectiveness of radium-223 dichloride (Ra-223) in patients with mCRPC in real-world setting: a Japanese post-marketing study (PMS). J Clin Oncol 38(Suppl 6):236
Tannock IF, de Wit R, Berry WR et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351(15):1502–1512
de Bono JS, Oudard S, Ozguroglu M et al (2010) Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376(9747):1147–1154
Hoskin P, Sartor O, O’Sullivan JM et al (2014) Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol 15(12):1397–1406
Vogelzang NJ, Coleman RE, Michalski JM et al (2017) Hematologic Safety of Radium-223 Dichloride: Baseline Prognostic Factors Associated With Myelosuppression in the ALSYMPCA Trial. Clin Genitourin Cancer 15(1):42-52.e8
Dizdarevic S, Petersen PM, Essler M et al (2019) Interim analysis of the REASSURE (Radium-223 alpha Emitter Agent in non-intervention Safety Study in mCRPC popUlation for long-teRm Evaluation) study: patient characteristics and safety according to prior use of chemotherapy in routine clinical practice. Eur J Nucl Med Mol Imaging 46(5):1102–1110
Matsubara N, Nagamori S, Wakumoto Y et al (2018) Phase II study of radium-223 dichloride in Japanese patients with symptomatic castration-resistant prostate cancer. Int J Clin Oncol 23(1):173–180
Scher HI, Morris MJ, Stadler WM et al (2016) Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol 34(12):14021418
Kakehi Y, Sugimoto M, Taoka R, Committee for establishment of the evidenced-based clinical practice guideline for prostate cancer of the Japanese Urological Association (2017) evidenced-based clinical practice guideline for prostate cancer. Int J Urol 24(9):648–666
National Comprehensive Cancer Network (NCCN) Guidelines for Patients. Prostate Cancer. 2020 version 1. Available from: https://www.nccn.org/patients/guidelines/content/PDF/prostate-patient.pdf. Accessed 12 June 2020
Sartor O, Hoskin P, Coleman RE et al (2016) Chemotherapy following radium-223 dichloride treatment in ALSYMPCA. Prostate 76(10):905–916
Acknowledgements
Data management and statistical analysis were performed by CMIC Co., Ltd. Preparation of the manuscript was supported by Kathy Croom and David P. Figgitt PhD, ISMPP CMPP™, Content Ed Net, and Haruka Kakiuchi, Bayer Yakuhin, Ltd. Medical writing support was funded by Bayer Yakuhin, Ltd.
Author information
Authors and Affiliations
Contributions
HU, NM, ST, MH, and SK were involved in the collection and interpretation of data. YK and TH was involved in the interpretation of data. YO was accountable for conducting and managing this study. TS was accountable for some analysis and interpretation of the data. All authors were involved in reviewing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
This study has been conducted by Bayer Yakuhin, Ltd. HU has received honoraria from Bayer Yakuhin, Ltd., Pfizer, Janssen Pharmaceutical K.K., Bristol-Myers Squibb, Merck Sharp & Dohme, and Chugai Pharmaceutical Co. Ltd, and research funding from AstraZeneca, Janssen Pharmaceutical K.K., Takeda Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Sanofi, and Daiichi Sankyo Co. Ltd. NM has received honoraria from Kissei Pharmaceutical Co. Ltd, Janssen Pharmaceutical K.K., Takeda Pharmaceutical Co. Ltd., Astellas Pharma Inc., and AstraZeneca, and research funding from Astellas Pharma Inc., and Ono Pharmaceutical Co. Ltd. ST has received honoraria from Bayer Yakuhin, Ltd., Merck Sharp & Dohme, AstraZeneca, Chugai Pharmaceutical Co. Ltd, Ono Pharmaceutical Co. Ltd., and Bristol-Myers Squibb, and research funding from Bayer Yakuhin, Ltd., Merck Sharp & Dohme, AstraZeneca, Chugai Pharmaceutical Co. Ltd, Ono Pharmaceutical Co. Ltd., and Bristol-Myers Squibb. MH has received honoraria from Bayer Yakuhin, Ltd. SK and YK declare no conflict of interest. TS, TH, and YO are employees of Bayer Yakuhin, Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Uemura, H., Masumori, N., Takahashi, S. et al. Real-world safety and effectiveness of radium-223 in Japanese patients with castration-resistant prostate cancer (CRPC) and bone metastasis: exploratory analysis, based on the results of post-marketing surveillance, according to prior chemotherapy status and in patients without concomitant use of second-generation androgen-receptor axis-targeted agents. Int J Clin Oncol 26, 753–763 (2021). https://doi.org/10.1007/s10147-020-01850-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01850-3