Abstract
Background
For unresectable or recurrent advanced gastric adenocarcinoma (AGC), tri-weekly administration of nanoparticle albumin-bound paclitaxel (nab-PTX) at 260 mg/m2 achieved a response rate of 27.8% in a phase II trial in Japan. However, frequent neutropenia and peripheral neuropathy limit its use in clinical settings. We, thus, conducted a single-arm phase II trial to investigate the efficacy and safety of a reduced dose (220 mg/m2) of tri-weekly nab-PTX.
Methods
Eligible patients included those with AGC and ECOG performance status of 0–2 who had received one or more prior chemotherapy containing fluoropyrimidine regimens. A reduced dose of nab-PTX (220 mg/m2) was administered tri-weekly. The primary endpoint was response rate (RR). Secondary endpoints were overall survival (OS), progression-free survival (PFS), disease-control rate (DCR), incidence of adverse events, relative dose intensity (RDI) and proportion of patients receiving subsequent chemotherapy.
Results
Among 33 patients enrolled, 32 were treated with protocol therapy. RR was 3.1% [95% confidence interval (CI), 0–16.2%], which did not reach the protocol-specified threshold (p = 0.966). DCR was 37.5% (95% CI, 21.1–56.3%). Median OS and PFS were 6.3 (95% CI, 4.4–14.2) and 2.2 (95% CI, 1.8–3.1) months, respectively. RDI was 97.8%. Twenty (62.5%) patients received subsequent chemotherapy. Toxicity was relatively mild with the most common grade ≥ 3 adverse events being neutropenia (38%), anemia (13%), fatigue (19%), anorexia (16%), and peripheral neuropathy (13%).
Conclusion
Tri-weekly nab-PTX with a reduced dose (220 mg/m2) is not recommended for AGC in a second-line or later setting, despite demonstrating less toxicity than at 260 mg/m2.
Clinical trial registration
The OGSG1302 trial was registered with UMIN-CTR as UMIN000000714.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Combination of chemotherapy with platinum agents and fluoropyrimidine has been regarded as the standard of care in a first-line setting for unresectable or recurrent advanced gastric adenocarcinoma (AGC) [1], to which trastuzumab, an anti-HER2 monoclonal antibody, is added in HER2-positive cases [2, 3]. Recently, oral fluoropyrimidine including S-1 and capecitabine, has generally been utilized instead of infusional 5-FU because of their convenience and tolerability [4,5,6]. The combination of S-1 and cisplatin is now accepted as the standard regimen for first-line chemotherapy for patients with AGC in Japan, based on the result of the SPIRITS trial [4].
For second-line chemotherapy, until weekly solvent-based paclitaxel (sb-PTX) plus ramucirumab, an anti-VEGFR antibody, demonstrated superiority over sb-PTX alone [7], sb-PTX alone was widely utilized in this setting based on a phase III study in which sb-PTX showed comparable efficacy to CPT-11 with less toxicity [8]. This was also supported by promising results of several phase II studies, yielding overall response rates (RRs) that ranged from 16 to 27% and overall survival (OS) times of 5–11 months [8,9,10,11,12]. However, sb-PXT can cause hypersensitivity and anaphylactic reactions in certain patients, mostly because of polyethoxylated castor oil contained in it [13]. To use this drug safely, premedication with steroids and histamine H-2 blockers is generally required. Moreover, sb-PTX contains alcohol. These factors limit the general use of sb-PTX in a certain subset of AGC patients.
Nanoparticle albumin-bound paclitaxel (nab-PXT) is a novel, biologically interactive, nanometer-size albumin-bound paclitaxel particle initially developed to avoid the toxicities associated with polyethoxylated castor oil. It can be administered as a high dose of paclitaxel without premedication with steroids and histamine H-2 blockers. Furthermore, nab-PTX can be administered in only 30 min and used safely for alcohol-intolerant patients [14]. In clinical trials for metastatic breast cancer (MBC) as well as non-small-cell lung cancer, nab-PTX demonstrated efficacy equivalent to or exceeding that of sb-PTX [15,16,17]. In patients with AGC, a phase II trial in Japan showed the efficacy of tri-weekly nab-PTX at 260 mg/m2 without anti-allergic premedication, with an overall RR, the primary endpoint of this study, of 27.8% [15/54; 95% confidence interval (CI), 16.5–41.6%] and the median progression-free survival (PFS) and OS being 2.9 (95% CI, 2.4–3.6) and 9.2 (95% CI, 6.9–11.4) months, respectively [18]. However, relatively high toxicity was indicated, with the most common grade 3/4 toxicities being neutropenia (49.1%), leucopenia (20.0%), lymphopenia (10.9%) and chemotherapy-induced peripheral neuropathy (CIPN) (23.6%) [18]. Against this background, the optimal dosing that can minimize toxicity without sacrificing anticancer efficacy remains to be established.
A phase I trial of nab-PTX in patients with advanced solid tumors determined the maximum tolerated dose to be 300 mg/m2 [19]. In MBC, the dose of nab-PTX was initially set as 300 mg/m2 [20], and then deduced to 260 mg/m2 in the subsequent phase III Ca012 trial, where tri-weekly nab-PTX demonstrated significantly superior RR as well as a longer time to progression compared to the conventional sb-PTX at a dose of 175 mg/m2 [15]. This study also identified grade 3 or higher CIPN as a considerable adverse event of nab-PTX [15]. To reduce such toxicity, low-dose tri-weekly nab-PTX (160–175 mg/m2) was examined in several phase II studies for MBC, showing good overall RR (23–39.5%) without CIPN of grade 3 or higher [21, 22].
These results reveal the need to develop a low-dose nab-PTX regimen in AGC. To this end, we conducted a phase II trial to evaluate the efficacy and safety of low-dose tri-weekly nab-PTX (220 mg/m2) in AGC patients in second-line or later setting.
Patients and methods
Study objectives and design
This study was conducted in accordance with the international ethical recommendations stated in the Declaration of Helsinki. The protocol was approved by the institutional ethics committees of each participating hospital and registered in the University Hospital Medical Information Network (UMIN) database (ID000000714). Written informed consent was obtained from each patient before enrollment.
This was a non-randomized, multicenter phase II study for patients with AGC for whom more than one regimen including fluorinated pyrimidine antineoplastic agents had failed. The primary endpoint was RR, and the secondary endpoints were OS, PFS, time to treatment failure (TTF), disease-control rate (DCR), safety, relative dose intensity and proportion of patients who received subsequent therapy. This trial was carried out in accordance with the Japanese Classification of Gastric Carcinoma of the 14th edition from the Japanese Gastric Cancer Association.
Eligibility criteria
The eligibility criteria of this study were as follows; (1) histologically confirmed unresectable or recurrent gastric or esophagogastric junction adenocarcinoma; (2) history of failure of one or more prior chemotherapy containing fluoropyrimidine regimens for HER2-negative cases or both fluoropyrimidine and Trastuzumab for HER2-positive cases; (3) age 20–80 years; (4) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2; (5) one measurable lesion according to RECIST ver. 1.1 criteria as determined via computed tomography (CT) within 4 weeks before enrollment; (6) no previous treatment with PTX; (7) adequate organ function, including leukocyte count under 12,000 mm3, neutrophil count over 2,000 mm3, platelet count over 100,000 mm3, hemoglobin level over 9.0 g/dl, serum bilirubin level under 1.5 mg/dl, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels of under 100 or 200 IU/L of patients with liver metastasis, a serum creatinine level under 1.5 mg/dl; (8) expected to survive for at least 90 days from the date of registration; and (9) cases with the provision of informed consent.
Exclusion criteria were as follows: (1) with a history of severe drug sensitivity; (2) with infection or suspected infection with a fever over 38.0 °C; (3) serious complications, such as interstitial pneumonia or lung fibrosis, uncontrolled diabetes, or renal or hepatic failure; (4) suffering more than four bouts of diarrhea; (5) a history or complication of heart disease, for example, congestive heart failure, myocardial infarction, ischemic heart disease requiring treatment, arrhythmia or valvular disease; (6) active double cancer; (7) peripheral neuropathy over grade 2; (8) difficulty enrolling due to a psychiatric or neurological disorders; (9) brain metastasis; (10) positivity for HBs antigen or HCV antibody, and (11) the a presence of any other condition that would make the treatment unsafe.
Written informed consent was obtained from each patient before enrollment and the protocol was approved by the institutional ethics committee of each participating centers.
Study design
Treatment
nab-PTX was administered intravenously on an outpatient basis by a 30-min infusion at a dose of 220 mg/m2 on day 1 of each 21-day cycle. No premedication, such as steroid or antihistamine premedication, was administered. Treatment was continued until disease progression, unacceptable toxicity, or consent withdrawal.
Two dose-reduction levels (level 1, 180 mg/m2 and level 2, 150 mg/m2) and one dose escalation level (260 mg/m2) were implemented under the dose-reduction or escalation criteria: if the number of neutrophils was 1500/mm3 or more after the administration of 220 mg/m2 nab-PTX in the previous course and the dose-reduction criteria were not violated, the dose of nab-PTX could be increased up to 260 mg/m2 in the next course.
Follow-up
Patients underwent hematological tests and assessments of clinical symptoms at least once during each course of chemotherapy. However, in the first course, hematological tests were conducted on the 1st, 8th and 15th day. The severity of adverse drug reactions was judged in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Thoracoabdominal CT scans were repeated at least every 6 weeks (± 2 weeks) after treatment initiation and at the end of the treatment in this study. The objective disease status was assessed in accordance with the RECIST guidelines, version1.1. An independent review board organized by the Osaka Gastrointestinal Cancer Chemotherapy Study Group (OGSG) objectively identified treatment responses and drug-related adverse events.
Statistical analysis
RR was reported to be 27.8% (95% CI: 16.5–41.3) in a phase II study of nab-PTX (260 mg/m2, q3w) with almost the same objective as the present study [18] and 23% in a phase II study of PTX (210 mg/m2; 95% CI: 13–36%) [9]. The calculation of the sample size for the study was based on an expected response rate of 25% and a threshold response rate of 10%, using a one-sided alpha error of 0.05 and statistical power of 80%. The planned sample size was 35 patients, allowing for four patients dropping out.
The analysis focused on patients who were enrolled in this study and received at least one course of nab-PTX treatment. Background data were summarized as frequency with proportion for categorical variables, and median with range for continuous variables. The response rate was evaluated using exact binomial test. Confidence intervals of response rate and disease-control rate were estimated by the Clopper–Pearson method. OS, PFS and TTF were estimated using the Kaplan–Meier method and the 95% CIs for survival rate were calculated using Greenwood’s formula. p values less than 0.05 were considered statistically significant. All statistical analyses were performed with S-plus version 3.6.1 (R Foundation for Statistical Computing, Vienna Austria).
Results
Between April 2014 and December 2018, 33 patients with AGC and ECOG PS of 0–2 who had received one or more prior chemotherapy containing fluoropyrimidine regimens were enrolled from 10 institutions in Japan. As one patient withdrew consent before the initial treatment, 32 patients received the study treatment and were evaluated for clinical response and safety. The patients’ characteristics are listed in Table 1. Twenty-seven patients were male (84.4%) and the median age was 70 years (range 48–82). Most of the patients had an ECOG PS of 0 or 1, whereas ECOG PS 2 was seen in two patients (6%). Twenty patients involved advanced cases and 12 involved relapse. The stages at initial treatment of the relapse cases were stage II for 1 patient, stage III for 9, and stage IV for 2. Twenty-three patients (72%) were enrolled as second-line treatment and nine patients (28%) as third-line treatment.
Efficacy
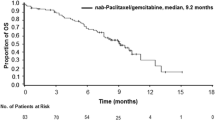
The overall responses in the 32 patients are summarized in Table 2. Partial response was achieved in only one patient, yielding an overall RR of 3.1% (95% CI, 0–16.2%), which did not reach the protocol-specified threshold (p = 0.966). Stable disease (SD) was observed in 11 patients, providing a DCR of 37.5% (95% CI, 21.1–56.3%). At the data cut-off (February 2019), the median follow-up was 6.3 months, and the number of treatment courses administered ranged from 1 to 27, with a median of 3. Only 1 out of 32 patients increased their treatment dose to 260 mg/m2 in the second course. The median PFS was 2.2 months (95% CI, 1.8–3.1) with the 6-month PFS rate being 9.4% (95% CI, 3.2–27.5%; Fig. 1). The median TTF was 2.0 months (95% CI, 1.8–3.0, Fig. 2). The RDI was 97.8% (average dose of 215 mg/m2). The median OS was 6.4 months (95% CI, 4.4–14.2) and the 1-year survival rate was 34.4% (95% CI, 21.3%–55.5%: Fig. 3). Subsequent chemotherapy was received by 20 of the 32 patients (62.5%; Table 3), in which the most commonly selected regimen was CPT-11-based chemotherapy (60%).
Toxicity
Grade 3 or 4 adverse events with an incidence rate of > 10% included neutropenia (38%), leucopenia (13%) and anemia (13%) as hematological toxicities, along with fatigue (19%), anorexia (16%), and CIPN (13%) as non-hematological toxicities. No patients experienced hypersensitivity or acute infusion reactions, although no premedication was administered at chemotherapy. Neither febrile neutropenia nor treatment-related deaths were observed (Table 4). The main reasons for treatment discontinuation or withdrawal were disease progression (26 cases: 81.3%) and adverse events (4 cases: 12.5%).
Discussion
In patients with AGC, the efficacy of tri-weekly nab-PTX at 260 mg/m2 without anti-allergic premedication was demonstrated with an overall RR of 27.8% in a phase II trial in Japan [18]. However, relatively high toxicities, such as neutropenia and CIPN, were indicated, which was the limitation of the general use of nab-PTX for patients with AGC. Therefore, we investigated the safety and efficacy of a reduced dose (220 mg/m2) of tri-weekly nab-PTX for patients with AGC with PS-0, 1 plus 2 in a second-line or later setting.
At the time when the current study was ongoing, a result of the phase III ABSOLUTE study was reported [23], in which the survival benefits of tri-weekly nab-PTX (260 mg/m2) or weekly nab-PTX (100 mg/m2) were compared with weekly sb-PTX (80 mg/m2) in patients with previously treated AGC. The results showed that weekly nab-PTX showed non-inferiority to weekly sb-PTX, whereas tri-weekly nab-PTX failed to demonstrate non-inferiority to sb-PTX. Interestingly, the study also showed that weekly nab-PTX had considerably lower grade 3 or higher toxicity than tri-weekly nab-PTX especially in terms of neutropenia (41.1 vs. 64.8%) and CIPN (2.5 vs. 20.1%). As a result of these findings, tri-weekly nab-PTX (260 mg/m2) is not commonly utilized in a clinical setting in AGC.
In the current study, we observed grade 3 or higher neutropenia at a rate of 38%, febrile neutropenia at 3%, and grade 3 or higher CIPN at 13%, whereas previous trials in AGC evaluating tri-weekly nab-PTX at 260 mg/m2 showed grade 3 or higher neutropenia and CIPN at rates of 49.1–64.8% and 20.1–23.6%, respectively. These results suggest that tri-weekly nab-PTX at 220 mg/m2 achieved the expected reduction of adverse events, consistent with previous trials in MBC [21, 22]
With regard to the efficacy, however, RR, the primary endpoint of this study, was only 3.1%, in addition to the relatively short median PFS and OS of 2.2 and 6.3 months, respectively. In contrast, previous studies evaluating tri-weekly nab-PTX at 260 mg/m2 showed RR of 25–27.8% as well as PFS and OS of 2.9–3.8 months and 9.2–10.3 months, respectively [18, 23]. These data indicate that the efficacy of the reduced dose of nab-PTX is not satisfactory, unlike in MBC [14, 20], despite the considerably low toxicities, so it is not recommended in clinical practice. Potential reasons for the poorer efficacy in the current study than in previous ones in AGC [18, 23] include the difference in the treatment line (second line or later vs. exclusively second line) and the relatively poor patients background in our study. In comparison with the other studies, our study population tended to be older (median age, 70 vs. 63.5 and 66 years) and had poorer ECOG PS (PS-0, 56 vs. 58.9 and 69%; as shown in Table 5). Indeed, the RDIs of phase II study and ABSOLUTE study were 93.4% (243 mg/m2) and 88.06% (229 mg/m2), respectively, which were higher than that in our study (97.8%: 215 mg/m2). Furthermore, the poorer patients’ background might lead to lower frequency of subsequent chemotherapy in the current study (62.5%) vs. others (81.5% [18] and 71% [23]) as was the number of patients administered CPT-11-based chemotherapy as subsequent therapy (53.7% and 51% [18] vs. 37.5% [23]). These results may support the hypothesis that the insufficient efficacy in the current study was derived from the inadequate treatment dose and the lower rate of subsequent treatment that may due to the difference in baseline patient characteristics between this study and the others.
In summary, the present study failed to show a clinical benefit of a reduced dose of tri-weekly nab-PTX in AGC and indicated that such treatment is not recommended, despite being potentially less toxic than the original dosing. Tri-weekly nab-PTX has little currency in the treatment of AGC as of the ABSOLUTE study [23], whereas the weekly administration of nab-PTX is now widely accepted. Following the success of the RAINBOW trial [7], weekly nab-PTX in combination with ramucirumab was examined in a phase II trial [24], showing promising activity with an RR of 54.8% and manageable toxicities. Given that the clinical benefit of weekly nab-PTX was pronounced in patients with peritoneal metastasis [23], a randomized phase II study comparing sb-PTX plus ramucirumab with nab-PTX plus ramucirumab is ongoing in AGC patients with peritoneal metastasis in a second-line setting (WJOG_P-SELECT study, jRCTs031180022), and the results of which are awaited.
References
Ohtsu A, Shimada Y, Shirao K et al (2003) Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group Study (JCOG9205). J Clin Oncol 21(1):54–59
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376(9742):687–697. https://doi.org/10.1016/S0140-6736(10)61121-X
Sawaki A, Ohashi Y, Omuro Y et al (2012) Efficacy of trastuzumab in Japanese patients with HER2-positive advanced gastric or gastroesophageal junction cancer: a subgroup analysis of the Trastuzumab for Gastric Cancer (ToGA) study. Gastric Cancer 15(3):313–322. https://doi.org/10.1007/s10120-011-0118-1
Koizumi W, Narahara H, Hara T et al (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9(3):215–221. https://doi.org/10.1016/S1470-2045(08)70035-4
Cunningham D, Starling N, Rao S et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358(1):36–46. https://doi.org/10.1056/NEJMoa073149
Kang YK, Kang WK, Shin DB et al (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 20(4):666–673. https://doi.org/10.1093/annonc/mdn717
Wilke H, Muro K, Van Cutsem E et al (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15(11):1224–1235. https://doi.org/10.1016/S1470-2045(14)70420-6
Hironaka S, Ueda S, Yasui H et al (2013) Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 Trial. J Clin Oncol 31(35):4438–4444. https://doi.org/10.1200/jco.2012.48.5805
Yamada Y, Shirao K, Ohtsu A et al (2001) Phase II trial of paclitaxel by three-hour infusion for advanced gastric cancer with short premedication for prophylaxis against paclitaxel-associated hypersensitivity reactions. Ann Oncol 12(8):1133–1137. https://doi.org/10.1023/a:1011680507956
Yamaguchi K, Tada M, Horikoshi N et al (2002) Phase II study of paclitaxel with 3-h infusion in patients with advanced gastric cancer. Gastric Cancer 5(2):90–95. https://doi.org/10.1007/s101200200015
Hironaka S, Zenda S, Boku N et al (2006) Weekly paclitaxel as second-line chemotherapy for advanced or recurrent gastric cancer. Gastric Cancer 9(1):14–18. https://doi.org/10.1007/s10120-005-0351-6
Kodera Y, Ito S, Mochizuki Y et al (2007) A phase II study of weekly paclitaxel as second-line chemotherapy for advanced gastric cancer (CCOG0302 study). Anticancer Res 27(4c):2667–2671
Gelderblom H, Verweij J, Nooter K et al (2001) Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 37(13):1590–1598. https://doi.org/10.1016/s0959-8049(01)00171-x
Henderson IC, Bhatia V (2007) Nab-paclitaxel for breast cancer: a new formulation with an improved safety profile and greater efficacy. Expert Rev Anticancer Ther 7(7):919–943. https://doi.org/10.1586/14737140.7.7.919
Gradishar WJ, Tjulandin S, Davidson N et al (2005) Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 23(31):7794–7803. https://doi.org/10.1200/jco.2005.04.937
Rizvi NA, Riely GJ, Azzoli CG et al (2008) Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non-small-cell lung cancer. J Clin Oncol 26(4):639–643. https://doi.org/10.1200/jco.2007.10.8605
Socinski MA, Bondarenko I, Karaseva NA et al (2012) Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 30(17):2055–2062. https://doi.org/10.1200/jco.2011.39.5848
Sasaki Y, Nishina T, Yasui H et al (2014) Phase II trial of nanoparticle albumin-bound paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Cancer Sci 105(7):812–817. https://doi.org/10.1111/cas.12419
Ibrahim NK, Desai N, Legha S et al (2002) Phase I and pharmacokinetic study of ABI-007, a cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res 8(5):1038–1044
Ibrahim NK, Samuels B, Page R et al (2005) Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol 23(25):6019–6026. https://doi.org/10.1200/jco.2005.11.013
Takashima T, Kawajiri H, Nishimori T et al (2018) Safety and efficacy of low-dose nanoparticle albumin-bound paclitaxel for HER2-negative metastatic breast cancer. Anticancer Res 38(1):379–383. https://doi.org/10.21873/anticanres.12233
Yamamoto S, Maeda N, Nagashima Y et al (2017) A phase II, multicenter, single-arm study of tri-weekly low-dose nanoparticle albumin-bound paclitaxel chemotherapy for patients with metastatic or recurrent breast cancer. Breast Cancer 24(6):783–789. https://doi.org/10.1007/s12282-017-0779-7
Shitara K, Takashima A, Fujitani K et al (2017) Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol 2(4):277–287. https://doi.org/10.1016/s2468-1253(16)30219-9
Bando H, Shimodaira H, Fujitani K et al (2018) A phase II study of nab-paclitaxel in combination with ramucirumab in patients with previously treated advanced gastric cancer. Eur J Cancer 91:86–91. https://doi.org/10.1016/j.ejca.2017.11.032
Acknowledgement
We thank all of the patients, investigators, and medical staff who participated in this study, as well as staff at the OGSG data center for their contribution.
Funding
This study was funded by Taiho Pharmaceutical Co., Ltd. under the study contract.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kazuhiro Nishikawa has received honoraria from Bristol-Myers Squibb, Chugai, EA Pharma, Eli Lilly, Ono, Taiho and Yakult. Hiroshi Imamura has received honoraria from Taiho. Kazumasa Fujitani has received honoraria from Bristol-Myers Squibb, Eli Lilly, Ono, Taiho and Yakult. Yukinori Kurokawa has received honoraria from Taiho, Ono, Eli Lilly, Yakult, Bristol-Myers Squibb, Kaken, Chugai and Takeda, and research funding from Taiho, Ono and MSD. Daisuke Sakai has received honoraria from Chugai and Diichi-Sankyo, and research funding from Chugai, Yakult, Ono, Eli Lilly and Diichi-Sankyo. Hisato Kawakami has received honoraria from Bristol-Myers Squibb. Astra-Zeneca, Bayer Yakuhin, Eli Lilly, MSD, Ono, Chugai, Daiichi Sankyo, Takeda and Taiho, and research funding from Chugai, Taiho and Eisai. Taroh Satoh has received honoraria from Bristol-Myers Squibb, Eli Lilly, Ono, Chugai and Taiho, and research funding from MSD, Gilead, Astellas and Astra-Zeneca, and have received scholarship donations from Taiho, and endowed chairs from Ono, Chugai and Yakult. All remaining authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tamura, S., Taniguchi, H., Nishikawa, K. et al. A phase II trial of dose-reduced nab-paclitaxel for patients with previously treated, advanced or recurrent gastric cancer (OGSG 1302). Int J Clin Oncol 25, 2035–2043 (2020). https://doi.org/10.1007/s10147-020-01768-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01768-w