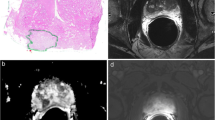
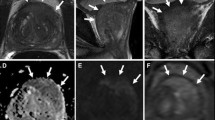
Abstract
Background
We investigated whether the detectability of prostate cancer with 3-Tesla (3T) multiparametric magnetic resonance imaging (mpMRI) differs by tumor location.
Methods
We identified 136 patients with prostate cancer who underwent 3-T mpMRI before prostatectomy at a single academic center. Two uroradiologists scored all MRIs with Prostate Imaging–Reporting and Data System version 2 (PI-RADS v2). A genitourinary pathologist mapped tumor foci from serial whole-mount radical prostatectomy sections. We assessed concordance of images with cancer sites. Tumor foci with Gleason score ≥ 3 + 4 or volume ≥ 0.5 mL were considered significant.
Results
A total of 122 foci in 106 cases were identified with mpMRI. Twenty-four were PI-RADS 3, 52 were 4, and 46 were 5. A total of 274 tumor foci were identified with whole-mount pathology. The sensitivity stratified by location to detect significant cancer with a PI-RADS cutoff value of 3 was 56.0% overall, 50.0% in the peripheral zone (PZ), 71.2% in the transitional zone (TZ), 62.4% anterior, 49.5% posterior, 42.0% apical, 63.6% in the midgland, and 43.8% in the gland base. In multivariate analysis, tumor location was not a significant predictor of identification by mpMRI. Tumor volume, Gleason score, and index tumor status were significantly associated with identification by mpMRI.
Conclusions
mpMRI detected the majority of high-grade and large cancers, but had low sensitivity in the PZ, posterior, and apex and base of the gland. The high prevalence of low-volume, low-Gleason score index tumors, as well as satellite tumors in those areas, accounted for the difference.


Similar content being viewed by others
References
Loeb S, Bjurlin MA, Nicholson J et al (2014) Overdiagnosis and overtreatment of prostate cancer. Eur Urol 65:1046–1055
Ahmed HU, El-Shater Bosaily A, Brown LC et al (2017) Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389:815–822
Ramsay E, Mougenot C, Staruch R et al (2017) Evaluation of focal ablation of magnetic resonance imaging defined prostate cancer using magnetic resonance imaging controlled transurethral ultrasound therapy with prostatectomy as the reference standard. J Urol 197:255–261
Schiavina R, Bianchi L, Borghesi M et al (2018) MRI displays the prostatic cancer anatomy and improves the bundles management before robot-assisted radical prostatectomy. J Endourol 32:315–321
Rud E, Baco E, Klotz D et al (2015) Does preoperative magnetic resonance imaging reduce the rate of positive surgical margins at radical prostatectomy in a randomised clinical trial? Eur Urol 68:487–496
Vargas HA, Hotker AM, Goldman DA et al (2016) Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol 26:1606–1612
Gaur S, Harmon S, Gupta RT et al (2019) A multireader exploratory evaluation of individual pulse sequence cancer detection on prostate multiparametric magnetic resonance imaging (MRI). Acad Radiol 26:5–14
Mirak SA, Shakeri S, Bajgiran AM et al (2019) Three Tesla multiparametric magnetic resonance imaging: comparison of performance with and without endorectal coil for prostate cancer detection, PI-RADS version 2 category and staging with whole mount histopathology correlation. J Urol 201:496–502
Tan N, Margolis DJ, Lu DY et al (2015) Characteristics of detected and missed prostate cancer foci on 3-T multiparametric MRI using an endorectal coil correlated with whole-mount thin-section histopathology. AJR Am J Roentgenol 205:W87–92
Greer MD, Brown AM, Shih JH et al (2017) Accuracy and agreement of PIRADSv2 for prostate cancer mpMRI: a multireader study. J Magn Reson Imaging 45:579–585
Le JD, Tan N, Shkolyar E et al (2015) Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol 67:569–576
Kenigsberg AP, Tamada T, Rosenkrantz AB et al (2018) Multiparametric magnetic resonance imaging identifies significant apical prostate cancers. BJU Int 121:239–243
Nix JW, Turkbey B, Hoang A et al (2012) Very distal apical prostate tumours: identification on multiparametric MRI at 3 Tesla. BJU Int 110:E694–700
Lewis S, Besa C, Rosen A et al (2017) Multiparametric magnetic resonance imaging for transition zone prostate cancer: essential findings, limitations, and future directions. Abdom Radiol (NY) 42:2732–2744
Rosenkrantz AB, Kim S, Campbell N et al (2015) Transition zone prostate cancer: revisiting the role of multiparametric MRI at 3 T. AJR Am J Roentgenol 204:W266–272
Weinreb JC, Barentsz JO, Choyke PL et al (2016) PI-RADS prostate imaging—reporting and data system: 2015, Version 2. Eur Urol 69:16–40
Kanda Y (2013) Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 48:452–458
Rosenkrantz AB, Verma S, Turkbey B (2015) Prostate cancer: top places where tumors hide on multiparametric MRI. AJR Am J Roentgenol 204:W449–456
Vargas HA, Akin O, Franiel T et al (2012) Normal central zone of the prostate and central zone involvement by prostate cancer: clinical and MR imaging implications. Radiology 262:894–902
Seles M, Gutschi T, Mayrhofer K et al (2016) Sampling of the anterior apical region results in increased cancer detection and upgrading in transrectal repeat saturation biopsy of the prostate. BJU Int 117:592–597
Borkowetz A, Platzek I, Toma M et al (2016) Direct comparison of multiparametric magnetic resonance imaging (MRI) results with final histopathology in patients with proven prostate cancer in MRI/ultrasonography-fusion biopsy. BJU Int 118:213–220
Dell'Oglio P, Stabile A, Soligo M et al (2019) there is no way to avoid systematic prostate biopsies in addition to multiparametric magnetic resonance imaging targeted biopsies. Eur Urol Oncol. https://doi.org/10.1016/j.euo.2019.03.002
Stabile A, Dell'Oglio P, De Cobelli F et al (2018) Association between prostate imaging reporting and data system (PI-RADS) score for the index lesion and multifocal, clinically significant prostate cancer. Eur Urol Oncol 1:29–36
Koizumi A, Narita S, Nara T et al (2018) Incidence and location of positive surgical margin among open, laparoscopic and robot-assisted radical prostatectomy in prostate cancer patients: a single institutional analysis. Jpn J Clin Oncol 48:765–770
Smith JA Jr, Chan RC, Chang SS et al (2007) A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol 178:2385–2389 (discussion 2389–2390)
Asvadi NH, Afshari Mirak S, Mohammadian Bajgiran A et al (2018) 3T multiparametric MR imaging, PIRADSv2-based detection of index prostate cancer lesions in the transition zone and the peripheral zone using whole mount histopathology as reference standard. Abdom Radiol (NY) 43:3117–3124
Bratan F, Niaf E, Melodelima C et al (2013) Influence of imaging and histological factors on prostate cancer detection and localisation on multiparametric MRI: a prospective study. Eur Radiol 23:2019–2029
Gawlitza J, Reiss-Zimmermann M, Thormer G et al (2017) Impact of the use of an endorectal coil for 3 T prostate MRI on image quality and cancer detection rate. Sci Rep 7:40640
Turkbey B, Merino MJ, Gallardo EC et al (2014) Comparison of endorectal coil and nonendorectal coil T2W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: correlation with whole-mount histopathology. J Magn Reson Imaging 39:1443–1448
Heijmink SW, Futterer JJ, Hambrock T et al (2007) Prostate cancer: body-array versus endorectal coil MR imaging at 3 T–comparison of image quality, localization, and staging performance. Radiology 244:184–195
Iremashvili V, Pelaez L, Manoharan M et al (2012) Tumor focality is not associated with biochemical outcome after radical prostatectomy. Prostate 72:762–768
Noguchi M, Stamey TA, McNeal JE et al (2003) Prognostic factors for multifocal prostate cancer in radical prostatectomy specimens: lack of significance of secondary cancers. J Urol 170:459–463
Priester A, Natarajan S, Khoshnoodi P et al (2017) Magnetic resonance imaging underestimation of prostate cancer geometry: use of patient specific molds to correlate images with whole mount pathology. J Urol 197:320–326
Lee T, Hoogenes J, Wright I et al (2017) Utility of preoperative 3 Tesla pelvic phased-array multiparametric magnetic resonance imaging in prediction of extracapsular extension and seminal vesicle invasion of prostate cancer and its impact on surgical margin status: experience at a Canadian academic tertiary care centre. Can Urol Assoc J 11:E174–E178
Acknowledgements
We thank Libby Cone, MD, MA, from Edanz Group Japan (www.edanzediting.com/ac) for editing drafts of this manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. The protocol for this study has been approved by the institutional Review Board of Kyoto University (#R1581).
Informed consent
Informed consent was obtained in the form of opt-out on the website from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ito, K., Furuta, A., Kido, A. et al. Detectability of prostate cancer in different parts of the gland with 3-Tesla multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Int J Clin Oncol 25, 732–740 (2020). https://doi.org/10.1007/s10147-019-01587-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01587-8