Abstract
Background
Most adolescents in Japan have recently been refraining from receiving the human papillomavirus (HPV) vaccine, following media reports of adverse medical events surrounding the vaccination and suspension of the Japanese governmental recommendation. We have previously reported that HPV vaccination of young girls is heavily influenced by guidance from their physicians concerning the vaccine and by the knowledge and attitude of the girls’ mothers towards cervical cancer. However, it has been unclear as to how the obstetricians and gynecologists were themselves affected by the negative media reports.
Methods
A questionnaire, including questions about their working status, attitudes toward HPV vaccination and about cervical cancer, and the HPV vaccination status of their daughters, was posted to obstetricians and gynecologists.
Results
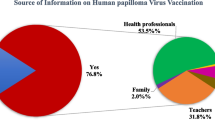
None of the daughters of the responding obstetrician and gynecologists received the HPV vaccination after the announced suspension of the governmental recommendation for the vaccine. The number who received the HPV vaccine in the 6th to 9th grade in 2014 was significantly lower than those in 2012 (p = 0.012). However, 64.7 % of the responders whose daughters were eligible and in the 6th to 12th grade still intended to vaccinate their daughters in the future. Of the responders, 65 % also intended to recommend vaccination to their teenage patients.
Conclusions
Our study revealed that obstetricians and gynecologists, like the general population, were negatively influenced by media reports of the adverse effect of the HPV vaccine and the suspension of the governmental recommendation. However, their intention to vaccinate their daughters was much higher than that of the general population. Restart of the governmental recommendation for HPV vaccines and better education about the HPV vaccine, including its adverse effects, and about cervical cancer and cervical cancer screening, are strongly recommended, for both the general public and for doctors, for improved prevention of cervical cancer.

Similar content being viewed by others
References
Lu B, Kumar A et al (2011) Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis. BMC Infect Dis 11:13
Garland SM, Hernandez-Avila M et al (2007) Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 356:1928–1943
Lehtinen M, Paavonen J et al (2012) Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 13:89–99
Paavonen J, Naud P et al (2009) Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 374:301–314
Darden PM, Thompson DM, Roberts JR et al (2013) Reasons for not vaccinating adolescents: National Immunization Survey of Teens, 2008–2010. Pediatrics 131:645–651
Ueda Y, Enomoto T, Sekine M et al (2015) Japan’s failure to vaccinate girls against human papilloma virus. Am J Obstet Gynecol 212:405–406
Morimoto A, Ueda Y, Egawa-Takata T et al (2014) Effect on HPV vaccination in Japan resulting from news report of adverse events and suspension of governmental recommendation for HPV vaccination. Int J Clin Oncol. doi:10.1007/s10147-014-0723-1
The Global Advisory Committee on Vaccine Safety (GACVS). GACVS Safety update on HPV vaccines (2013) http://www.who.int/vaccine_safety/committee/topics/hpv/130619HPV_VaccineGACVSstatement.pdf. Accessed Apr 2014
The Internaional Federation of Gynecology and Obstetrics (FIGO). FIGO Statement on HPV Vaccination Safety (2013) http://www.figo.org/files/figo-corp/Statement%20on%20Safety%20of%20HPV%20vaccination%20-%20FINAL%20-%20AUGUST%202013.pdf. Accessed Apr 2014
Japanese Ministry of Health, Labour and Welfare. A joint meeting of the Vaccine Adverse Reactions Review Committee (VARRC). http://www.mhlw.go.jp/stf/shingi/0000050385.html. Accessed 4 July 2014
Slade BA, Leidel L, Vellozzi C et al (2009) Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 302:750–757
Acknowledgments
We would like to thank Dr. G. S. Buzard for his constructive critiques and editing of our manuscript. We deeply appreciate Ms. Asami Yagi and Ms. Kanako Sakiyama for their support.
Conflict of interest
Authors Yutaka Ueda and Takayuki Enomoto receive lecture fee and research funding from Merck Sharp & Dohme (MSD.) and GlaxoSmithKline (GSK) to conduct a different study. Shinya Matsuzaki received research funding from MSD. Tadashi Kimura received a lecture fee from MSD and GSK.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Egawa-Takata, T., Ueda, Y., Morimoto, A. et al. Human papillomavirus vaccination of the daughters of obstetricians and gynecologists in Japan. Int J Clin Oncol 21, 53–58 (2016). https://doi.org/10.1007/s10147-015-0869-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0869-5