Abstract
Introduction: The pathogenesis of chronic subdural hematoma (CSDH) has not been completely understood. However, different mechanisms can result in space-occupying subdural fluid collections, one pathway can be the transformation of an original trauma-induced acute subdural hematoma (ASDH) into a CSDH. Materials and Methods: All patients with unilateral CSDH, requiring burr hole trephination between 2018 and 2023 were included. The population was distributed into an acute-to-chronic group (group A, n = 41) and into a conventional group (group B, n = 282). Clinical and radiographic parameters were analyzed. In analysis A, changes of parameters after trauma within group A are compared. In analysis B, parameters between the two groups before surgery were correlated. Results: In group A, volume and midline shift increased significantly during the progression from acute-to-chronic (p < 0.001, resp.). Clinical performance (modified Rankin scale, Glasgow Coma Scale) dropped significantly (p = 0.035, p < 0.001, resp.). Median time between trauma with ASDH and surgery for CSDH was 12 days. Patients treated up to the 12th day presented with larger volume of ASDH (p = 0.012). Before burr hole trephination, patients in group A presented with disturbance of consciousness (DOC) more often (p = 0.002), however less commonly with a new motor deficit (p = 0.014). Despite similar midline shift between the groups (p = 0.8), the maximal hematoma width was greater in group B (p < 0.001). Conclusion: If ASDH transforms to CSDH, treatment may become mandatory early due to increase in volume and midline shift. Close monitoring of these patients is crucial since DOC and rapid deterioration is common in this type of SDH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic subdural hematoma (CSDH) is one of the most frequent entities of intracranial hemorrhages [2, 26]. The pathogenesis of chronic subdural hematoma (CSDH) has not been completely understood. It usually develops from a mild head trauma which occurs within 6 months before clinical manifestation. However, atraumatic cases of CSDH have been reported as well [4, 7, 10, 18]. Another etiology of CSDHs is the development from an acute subdural hematoma (ASDH), which was managed conservatively initially and did not regress but liquefied and enlarged [1, 6, 9, 13, 16, 20]. Special attention should be given to this type of SDH since sudden clinical deterioration even with disturbance of consciousness (DOC) has been reported during the liquefaction phase [19]. Delayed surgery was reported to be necessary in only 6.5% of conservatively managed ASDH [3]. Risk factos which promote the development into a CSDH have been studied in multiple trials. Diabetes mellitus, use of antiplatelet or anticoagulative drugs, larger ASDH volume and midline shift are some of the risk factors for the development into a CSDH [9, 13, 16, 20]. On the one hand, identifying patients at risk is beneficial for clinical monitoring of these ASDH patients, and on the other hand, knowledge about the clinical and radiographic progression from ASDH to CSDH within the days and weeks after trauma is important for monitoring and early adequate treatment of these patients. The course of these patients has only been reported in some small case series [1, 6, 15, 19]. Also, limited data is available about potential differences of this form of CSDH and the conventional CSDH form which usually develops over a longer period of time [7, 10].
Despite the low number of reported cases with this phenomenon, special awareness should be given due to the sudden clinical deterioration. Therefore, our study aimed to analyze radiographic and clinical progression from ASDH to CSDH after trauma and risk factors for early need for burr hole trephination in this form of CSDH (acute-to-chronic SDH).
Materials and methods
Patient selection and study design
A retrospective data acquisition was conducted for consecutive unilateral CSDH patients who were treated by burr hole trephination and subdural drainage in our department from January 2018 to December 2023. The study cohort was divided into an acute-to-chronic group (group A) and into a conventional group (group B). In group A, patients were included who evidently developed the CSDH from an ASDH. Patients without the demonstration of an ASDH were included in group B (Fig. 1).
The primary endpoint of this study was the volume change of SDH in group A during liquefaction phase. Secondary endpoints were time in days between trauma and surgery in group A, midline shift, maximal hematoma width, modified Rankin Scale (mRS), Glasgow Coma Scale (GCS), new motor deficit, disturbance of consciousness (DOC), epileptic seizure, death during hospital stay and recurrence within 8 weeks in group A and B. DOC in patients was defined as a drop of GCS of at least 3 points.
Exclusion criteria were the following:
-
patients with recurrent or bilateral CSDHs.
-
CSDH of the posterior fossa.
-
patients under 18 years of age.
-
CSDH patients who were treated by embolization.
-
patients with ASDH who were treated by craniotomy or without the need for surgical treatment due to regressive subdural hematoma.
-
patients with Glasgow Coma Scale (GCS) ≤ 8 on initial presentation with ASDH.
-
atraumatic ASDH.
-
patients with significant additional intracranial bleeding such as epidural hematoma, significant traumatic subarachnoid hemorrhage, traumatic intraparenchymal hematoma or with bilateral ASDH.
Data acquisition
All data were retrospectively obtained from medical reports, radiological reports and cranial CT-imaging. The overall health status was summarized with the Charlson Comorbidity Index [5]. Radiographic data for both groups were taken from the preoperative CT scans and included the midline shift, hematoma width and locations of CSDH over the cerebral hemisphere. Location was divided into frontal, parietal, temporal and occipital location. Midline shift and hematoma width were measured on axial CT scan and the maximum was recorded. Additionally, volume of ASDH and CSDH in group A was measured by the software Visage Imaging VISAGE 7 Version 7.1.15. Maximal density of the ASDH and CSDH in Hounsfield Units (HU) was also measured as a sign of liquefaction of the hematoma. If ASDH enlarged, initial CT scan was taken with maximal volume of ASDH.
Indication for treatment and postoperative CT scans
Indication for burr hole trephination was given for patients due to worsening of clinical symptoms and a verified sufficient liquefaction of the hematoma on CT-scan. Other patients, who were treated by craniotomy or remained with conservative treatment, were excluded from this study.
The first postoperative CT scan was conducted on the second postoperative day. A follow-up CT scan was scheduled three to four weeks after burr hole trephination. CT scan was also conducted on clinical deterioration.
Antiplatelet and anticoagulative drugs were discontinued in all patients at admission. Anticoagulative drugs were reversed with prothrombin complex and vitamin K. Antiplatelet drugs were not counteracted, neither with platelet transfusion nor desmopressin, in this patient cohort since this is only conducted in acute craniotomies in our department. Prophylaxis for thromboembolism was conducted in all patients with a low-molecular-weight heparin or unfractioned heparin in case of renal failure. Administration of prophylactic drug was started one day after trauma and discontinued for the day of surgery.
Statistical analysis and ethics
Statistical analysis was performed with IBM SPSS-Software Version 27.
In the analysis A, clinical and radiographic data within group A was analyzed (Fig. 1). Patients clinical and radiographic parameters were compared between initial presentation with ASDH and the preoperative CT scan and presentation before surgery for CSDH. For comparison of these paired samples, the Wilcoxon rank sum test and the Wilcoxon signed-rank test were applied. For subgroup analyses within group A, the Mann-Whitney U-test was performed.
In the analysis B, clinical and radiographic parameters before burr hole trephination between group A and B were compared (Fig. 1). For this bivariate analysis, categorial variables were tested with the Pearson Chi square test or with the Fisher’s exact test. Odds ratios (OR) and 95% Confidence intervals (CI) are given for dichotomous variables which were significant. Continuous variables were first tested for normal distribution with the Kolmogorov-Smirnov test. Then the Mann-Whitney-U test was performed. The continuous variables are given as mean and standard deviation (SD).
Significance level of < 0.05 was considered statistically significant in two-sided testing.
The study was approved by the Institutional Review Board (IRB-2023-15). Due to its retrospective design, informed consent was waived.
Results
Patient cohort
323 patients were included in this study. 41 patients evidently developed the CSDH from an ASDH (group A) (Fig. 1). 13 subacute SDH patients were excluded from the study since they required delayed craniotomy (at least 48 h after trauma) rather than burr hole trephination.
Mean age was not different between the groups (both 77.1 years) and the median Charlson Comorbidity Index was 4 in both groups.
Analysis A
Progression of radiographic and clinical parameters
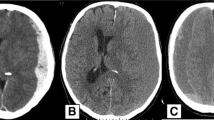
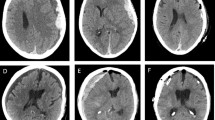
Both, volume of subdural hematoma and midline shift during liquefaction phase increased significantly (mean 50.4 to 95.5 ml, mean 4.6 to 9.4 mm, p < 0.001, resp.) (Fig. 2). As expected during this period, maximal density in HU dropped significantly as well (p < 0.001) (Table 1). 20/41 of ASDHs spread along the entire hemisphere on the initial CT scan.
Subsequently, clinical performance summarized in the above-mentioned scores dropped significantly. Median mRS (1 to 2, p = 0.035) and GCS (15 to 13, p < 0.001) decreased during the liquefaction phase. (Table 1).
12 patients presented with DOC before surgery for CSDH, three of them with GCS < 8. Patients with DOC before surgery had a significantly higher midline shift but not a larger volume with the ASDH on the initial CT scan (5.9 vs. 4 mm, p = 0.025; 57.2 vs. 47.6 ml, p = 0.357; resp.)
Six cases in group A had to undergo second hematoma evacuation via burr hole trephination after a median time of 15 days after initial surgery.
Factors influencing time between initial ASDH and surgery for CSDH
Median time between trauma with evidence of ASDH and surgery for CSDH was 12 days (SD 7.7, max. 38, min. 5). 23 patients (56%) were treated up to day 12. Number of patients treated over time after trauma is outlined in Fig. 3.
Patients treated up to the 12th day presented with a significantly larger initial volume of ASDH (59.5 vs. 38.8 ml, p = 0.012). Patients with more than 5 mm midline shift (n = 18) and/or with ASDH volume of more than 50 ml (n = 20) were treated earlier (10.5 vs. 16 days, p = 0.021; 11 vs. 16 days, p = 0.017; resp.).
Neither antiplatelet nor anticoagulative drugs had an impact on the time period from ASDH to burr hole trephination (p = 0.767, p = 0.722; resp.).
Analysis B
In the comparative analysis between both groups, no difference in median mRS (both 2, p = 0.58) was evident. However, patients in group A presented with DOC significantly more often (p = 0.002), but less commonly with a new motor deficit (p = 0.014). Epileptic seizures were not more frequent in either of the groups (p = 1) (Table 2).
Concerning the radiographic data, frontal and parietal location were not different between the groups (both p = 1), but distribution of the hematoma with additional temporal and occipital locations were more common in group A (both p < 0.001). Hematomas with complete hemispheric distribution, over all lobes, was much more common in group A (p < 0.001) (Table 2).
Despite similar midline shift between the groups (8.8 vs. 8.5 mm, p = 0.8), the maximal hematoma width was greater in group B (15.5 vs. 22.4 mm, p < 0.001) (Table 2).
Discussion
In our study we analyzed the progression from ASDH to CSDH over time after trauma. The median number of days was 12 and patients with larger volume of ASDH tend to be treated earlier for CSDH. Clinical performance of patients worsened during the liquefaction course and DOC was not uncommon in this group, especially in patients with larger initial midline shift.
The path of ASDH is obvious in most cases with indication for acute evacuation via craniotomy or craniectomy in patients with lower GCS, however, in patients with initial conservative therapy and clinical monitoring, delayed craniotomy or burr hole trephination might be necessary due to expansion of the hematoma and/or clinical deterioration later on [22, 24, 28].
When awaiting liquefaction of ASDH in order to treat it with minimal approach via burr hole trephination, increase of hematoma size and midline shift while worsening clinical performance with drop of GCS should be monitored since it has been reported not only in our study [1, 6, 11]. Additionally, patients with ASDH who were initially managed conservatively were also more likely to be readmitted within 30 days due to SDH after discharge [8].
In our study the median time between trauma with ASDH and surgery for CSDH was 12 days. Kpealo et al. reported onset of symptoms after trauma with ASDH after 27.4 days in case of CSDH and 12.6 days in case of subacute SDH [19]. In a study by Kang et al., 9 patients with SDH in the subacute stage were treated via burr hole trephination and injection of urokinase in patients with increased midline shift or clinical deterioration after 10.2 days of trauma with ASDH [15]. Other studies reported similar time periods with mean number of days between 11 and 18 after trauma with ASDH and burr hole trephination for liquefied subdural hematoma [1, 6, 13].
So, our results are in accordance with the previously published literature. As outlined in Fig. 3, most patients in group A were treated between day 9 and 19. Radiographic parameters of ASDH could help to establish a schedule for follow up CT scans for conservatively managed ASDH, since in our study, patients with larger hematoma volume (esp. > 50 ml) and midline shift (esp. > 5 mm) of ASDH on the initial CT scan, presented earlier for burr hole trephination. As already known, larger hematoma size and midline shift seems to be predictive for the development of a CSDH from ASDH [9, 16]. However, the impact of ASDH volume and midline shift on outcome seems to be ambiguous still [22]. Patients with higher midline shift with ASDH also presented more common with DOC at the time of burr hole trephination in our study. 3 patients presented with a GCS < 8, whereas the other nine patients with DOC had a higher GCS.
Despite, the use of antiplatelet and anticoagulative drugs have been shown to promote chronification of ASDH, our analysis did not find a faster progression to CSDH [9, 13]. However, it must be mentioned that these drugs were discontinued and in case of coumarins antagonized. But the antiplatelet and anticoagulative drugs’ effects continue to act for a couple of days [14, 21, 25, 27]. This delayed effect did not seem to impact the time of progression in our patient cohort, though. Concerning prophylaxis for thromboembolism, early prophylaxis (< 48 h after trauma) in patients with severe SDH was reported to be safe and more effective in preventing thromboembolic events than late prophylaxis [12]. In our patient cohort, prophylaxis was started after 24 h. It must be mentioned that stable clinical and/or radiographic status should be documented before starting prophylaxis for thromboembolism. Concerning the timing of the prophylaxis, each case should be evaluated individually based on the clinical and radiographic status as well as the risk profile for thromboembolism.
When we compared this type of SDH with the conventional CSDH which develops over a couple of weeks or even months, we concluded, that acute-to-chronic SDH are symptomatic and require surgery with less hematoma size but with similar midline shift. This might be due to the faster development of CSDH from ASDH, with a median number of days of only 12 in our study, whereas conventional CSDH usually develops over a couple of weeks or even months with the formation of two membranes within the subdural space [7, 10]. This slower development of mass effect of the hematoma in conventional CSDH probably leads to a larger hematoma size without more extensive midline shift due to possible brain parenchymal compliance, especially in elderly [17]. This faster development in the acute-to-chronic entity might be also the reason for higher incidence of DOC compared to conventional type. Kpealo et al. already stressed the dangerousness of sudden drop in GCS in the liquefaction course from ASDH [19].
Motor deficit was more common in the conventional group. This might be due to higher maximal hematoma size as this factor was reported as a predictor for motor deficit before [23]. In our study, it might also be possible that DOC in the acute-to-chronic group concealed the motor deficit.
In the acute-to-chronic SDH additional temporal and occipital as well as complete distribution over one hemisphere was much more common than in the conventional group. However, this is most likely due to the wider spread over the hemisphere of the ASDH. 20/41 of ASDHs were spread along the entire hemisphere. Little is known in the literature about the distribution of the CSDH over the cerebral hemisphere and its impact. Our results indicate that acute-to-chronic SDH spreads more commonly over the entire hemisphere due to wider spread of initial ASDH.
Strengths and limitations
Some limitations need to be addressed in this study. Due to its retrospective design a strict standardized schedule for control CT scans in group A was not achieved, clinical assessment could also be just taken from medical reports. Another limitation is the possibility that some CSDH might have also developed from ASDH in group B, even though that patients’ history was not suggestive for this.
Strengths of this study is the volume assessment via software in group A. Volume assessment was performed via a software rather than by manual calculation. The detailed analysis of radiographic and clinical parameters over time in both groups is another strength. We also report a rather high sample size of patients with the acute-to-chronic SDH form.
Conclusion
If ASDH develops to CSDH, treatment is necessary earlier due to increase in volume and midline shift. Patients with larger ASDH volume tend to be treated even earlier. Therefore, close monitoring of these patients is crucial since sudden clinical deterioration even with DOC is not uncommon in this type of SDH.
Data availability
Data is provided within the manuscript and its tables.
Abbreviations
- CSDH:
-
Chronic subdural hematoma
- ASDH:
-
Acute subdural hematoma
- SDH:
-
Subdural hematoma
- GCS:
-
Glasgow coma scale
- mRS:
-
Modified Rankin Scale
- DOC:
-
Disturbance of consciousness
References
Akbik OS, Starling R, Green R, Zhu Y, Lewis J (2020) Delayed Surgical Intervention in Acute Subdural Hematoma. Cureus. https://doi.org/10.7759/cureus.11592
Baiser D, Farooq S, Mehmood T, Reyes M, Samadani U (2015) Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J Neurosurg 123:1209–1215. https://doi.org/10.3171/2014.9.JNS141550
Bajsarowicz P, Prakash I, Lamoureux J, Saluja RS, Feyz M, Maleki M, Marcoux J (2015) Nonsurgical acute traumatic subdural hematoma: what is the risk? J Neurosurg 123:1176–1183. https://doi.org/10.3171/2014.10.JNS141728
Blaauw J, Meelis GA, Jacobs B, van der Gaag NA, Jellema K, Kho KH, Groen RJM, van der Naalt J, Lingsma HF, den Hertog HM (2022) Presenting symptoms and functional outcome of chronic subdural hematoma patients. Acta Neurol Scand 145:38–46. https://doi.org/10.1111/ane.13518
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Choi YH, Han SR, Lee CH, Choi CY, Sohn MJ, Lee CH (2017) Delayed burr hole surgery in patients with acute subdural hematoma: clinical analysis. J Korean Neurosurg Soc 60:717–722. https://doi.org/10.3340/jkns.2017.0404.010
Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ (2017) Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation 14
Franko LR, Sheehan KM, Roark CD, Joseph JR, Burke JF, Rajajee V, Williamson CA (2018) A propensity score analysis of the impact of surgical intervention on unexpected 30-day readmission following admission for subdural hematoma. Journal of Neurosurgery. American Association of Neurological Surgeons, pp 1008–1016
Gaonkar VB, Garg K, Agrawal D, Chandra PS, Kale SS (2021) Risk factors for progression of conservatively managed Acute traumatic subdural hematoma: a systematic review and Meta-analysis. World Neurosurg 146:332–341
Holl DC, Volovici V, Dirven CMF, Peul WC, van Kooten F, Jellema K, van der Gaag NA, Miah IP, Kho KH, den Hertog HM, Lingsma HF, Dammers R (2018) Pathophysiology and nonsurgical treatment of chronic subdural hematoma: from past to Present to Future. World Neurosurg 116:402–411e2
Izumihara A, Yamashita K, Murakami T (2012) Acute Subdural Hematoma Requiring Surgery in the Subacute or Chronic Stage
Jakob DA, Benjamin ER, Recinos G, Cremonini C, Lewis M, Demetriades D (2022) Venous thromboembolic pharmacological prophylaxis in severe traumatic acute subdural hematomas: early prophylaxis is effective and safe. Am J Surg 223:1004–1009. https://doi.org/10.1016/j.amjsurg.2021.07.048
Kaestner S, Van Den Boom M, Deinsberger W (2019) Frequency of and risk factors for chronification in traumatic acute subdural hematoma following conservative therapy. J Neurol Surg Cent Eur Neurosurg 80:359–364. https://doi.org/10.1055/s-0039-1685188
Kampouraki E, Wynne H, Avery P, Kamali F (2020) Validation of an algorithm to predict decline in INR following warfarin cessation in patients undergoing invasive procedures. J Thromb Thrombolysis 49:630–635. https://doi.org/10.1007/s11239-019-02017-2
Kang HG, Cho KY, Lee RS, Lim JS (2019) Delayed operation of acute subdural hematoma in subacute stage by trephine drainage using urokinase. Korean J Neurotrauma 15:103–109. https://doi.org/10.13004/kjnt.2019.15.e32
Kayahara T, Kikkawa Y, Komine H, Kamide T, Suzuki K, Shibata A, Ikeda S, Ikeda T, Kurita H (2020) Predictors of subacute hematoma expansion requiring surgical evacuation after initial conservative treatment in patients with acute subdural hematoma. Acta Neurochir (Wien) 162:357–363. https://doi.org/10.1007/s00701-019-04187-7
Khorasanizadeh M, Paul U, Chang Y-M, Moore JM, Ogilvy CS, Thomas AJ (2023) The effect of patient age on the degree of midline shift caused by chronic subdural hematomas: a volumetric analysis. J Neurosurg 1–7. https://doi.org/10.3171/2023.6.jns222890
Kostić A, Kehayov I, Stojanović N, Nikolov V, Kitov B, Milošević P, Kostić E, Zhelyazkov H (2018) Spontaneous chronic subdural hematoma in elderly people – arterial hypertension and other risk factors. J Chin Med Association 81:781–786. https://doi.org/10.1016/j.jcma.2018.03.010
Kpelao E, Beketi KA, Moumouni AK, Doleagbenou A, Ntimon B, Egbohou P, Mouzou T, Tomta K, Sama DH, Abalo A, Walla A, Dossim A (2016) Clinical profile of subdural hematomas: dangerousness of subdural subacute hematoma. Neurosurg Rev 39:237–240. https://doi.org/10.1007/s10143-015-0669-4
Kwon H, Choi KS, Yi HJ, Chun HJ, Lee YJ, Kim DW (2017) Risk factors of delayed surgical intervention after conservatively treated acute traumatic subdural hematoma. J Korean Neurosurg Soc 60:723–729. https://doi.org/10.3340/jkns.2017.0506.011
Lee J, Kim JK, Kim JH, Dunuu T, Park S-H, Park SJ, Kang JY, Choi RK, Hyon MS (2014) Recovery time of platelet function after aspirin withdrawal. Curr Therapeutic Res 76:26–31. https://doi.org/10.1016/j.curtheres.2014.02.002
Manivannan S, Spencer R, Marei O, Mayo I, Elalfy O, Martin J, Zaben M (2021) Acute subdural haematoma in the elderly: To operate or not to operate? A systematic review and meta-analysis of outcomes following surgery. BMJ Open 11
Motiei-Langroudi R, Alterman RL, Stippler M, Phan K, Alturki AY, Papavassiliou E, Kasper EM, Arle J, Ogilvy CS, Thomas AJ (2019) Factors influencing the presence of hemiparesis in chronic subdural hematoma. J Neurosurg 131:1926–1930. https://doi.org/10.3171/2018.8.JNS18579
Phan K, Moore JM, Griessenauer C, Dmytriw AA, Scherman DB, Sheik-Ali S, Adeeb N, Ogilvy CS, Thomas A, Rosenfeld JV (2017) Craniotomy Versus Decompressive Craniectomy for Acute Subdural Hematoma: systematic review and Meta-analysis. World Neurosurg 101:677–685e2. https://doi.org/10.1016/j.wneu.2017.03.024
Price MJ, Walder JS, Baker BA, Heiselman DE, Jakubowski JA, Logan DK, Winters KJ, Li W, Angiolillo DJ (2012) Recovery of platelet function after discontinuation of prasugrel or clopidogrel maintenance dosing in aspirin-treated patients with stable coronary disease. J Am Coll Cardiol 59:2338–2343. https://doi.org/10.1016/j.jacc.2012.02.042
Rauhala M, Luoto TM, Huhtala H, Iverson GL, Niskakangas T, Öhman J, Helén P (2020) The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. Journal of Neurosurgery. American Association of Neurological Surgeons, pp 1147–1157
Söderberg M, Holm M, Gupta A (2020) Assessment of platelet function after discontinuation of ticagrelor. Acta Anaesthesiol Scand 64:526–531. https://doi.org/10.1111/aas.13524
Vega RA, Valadka AB (2017) Natural history of Acute Subdural Hematoma. Neurosurg Clin N Am 28:247–255. https://doi.org/10.1016/j.nec.2016.11.007
Funding
Open access funding provided by Paracelsus Medical University. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Open access funding provided by Paracelsus Medical University.
Author information
Authors and Affiliations
Contributions
A.L. designed the concept, performed data collection and analysis, wrote the main manuscript text and prepared the figures and tables.E.H. helped to design the concept, performed data collection and analysis and reviewed the manuscript.T.E. helped to design the concept, reviewed data analysis and the manuscript.A.H. reviewed data analysis and the manuscript.H.S. reviewed the manuscript.K.S. designed the concept, reviewed the manuscript and supervised the project.L.R. designed the concept, helped to write the manuscript text and create figures and tables, and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the institutional review board (IRB-2023-15). Due to its retrospective design, informed consent was waived.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liebert, A., Hirschmann, E., Eibl, T. et al. Acute-to-chronic subdural hematoma: radiographic and clinical progression from acute subdural hematoma. Neurosurg Rev 47, 247 (2024). https://doi.org/10.1007/s10143-024-02465-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-024-02465-2