Abstract
A major challenge within the academic literature on SDHs has been inconsistent outcomes reported across studies. Historically, patients have been categorized by the blood-product age identified on imaging (i.e., acute, subacute, or chronic). However, this schematic has likely played a central role in producing the heterogeneity encountered in the literature. In this investigation, a total of 494 patients that underwent SDH evacuation at a tertiary medical center between November 2013-December 2021 were retrospectively identified. Mechanism of injury was reviewed by the authors and categorized as either positive or negative for a high-velocity impact (HVI) injury. Any head strike injury leading to the formation of a SDH while traveling at a velocity beyond that of normal locomotion or daily activities was categorized as an HVI. Patients were subsequently stratified by those with an acute SDHs after a high-velocity impact (aSDHHVI), those with an acute SDH without a high-velocity impact injury (aSDHWO), and those with any combination of subacute or chronic blood products (mixed-SDH [mSDH]). Nine percent (n = 44) of patients experienced an aSDHHVI, 23% (n = 113) aSDHWO, and 68% (n = 337) mSDH. Between these groups, highly distinct patient populations were identified using several metrics for comparison. Most notably, aSDHHVI had a significantly worse neurological status at discharge (50% vs. 23% aSDHWO vs. 8% mSDH; p < 0.001) and mortality (25% vs. 8% aSDHWO vs. 4% mSDH; p < 0.001). Controlling for gender, midline shift (mm), and anticoagulation use in the acute SDH population, multivariable logistic regression revealed a 6.85x odds ratio (p < 0.001) for poor outcomes in those with a positive history for a high-velocity impact injury. As such, the distribution of patients that suffer an HVI related acute SDH versus those that do not can significantly affect the outcomes reported. Adoption of this stratification system will help address the heterogeneity of SDH reporting in the literature while still closely aligning with conventional reporting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
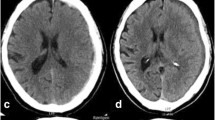
Subdural hematomas (SDH) represent one of the most common intracranial mass-lesions associated with high morbidity and mortality [1]. Predominately a disease of the elderly, recent projections indicate 1.5 billion individuals will be over 65 by 2050, with over 80 representing the fastest growing segment of the population in developed countries [2]. Historically, SDHs have been categorized by the blood product age, i.e., acute, subacute, and chronic (Fig. 1) [3, 4]. However, the differences that exist between SDH types are not limited to exclusively their radiographic densities, which may have only a small role in the complex pathophysiology pathway from which they develop. The majority of SDHs occur secondary to traumatic brain injury (TBI) [5]. Traumatic brain injuries can vary greatly in both their mechanism of injury and severity. Herein arises the challenge in categorizing these varied patients. Despite sharing blood products identified on computed tomography (CT), conventional reporting does not allot any measure towards the magnitude of TBI. Traumatic brain injuries unto themselves are associated with numerous physiologic changes and downstream sequalae with the potential to have devastating neurological outcomes. Although a SDH is often easily recognized on imaging, it still does not adequately represent the underlying injury which may be occurring on a molecular or cellular level [6]. Consequently, an assortment of intracranial pathology is frequently categorized together under a single designation.
Conventional categorization of subdural hematomas. In this panel of computed tomography coronal images, the three types of subdural hematomas are illustrated using conventional reporting. Figure 1A shows an acute subdural hematoma with a hyperdense appearance relative to brain tissue while a subacute subdural hematoma is isodense (Fig. 1B). A chronic subdural hematoma is hypodense relative brain tissue and similar in appearance to cerebrospinal fluid (Fig. 1C)
A major challenge within the academic literature on SDHs has been inconsistent outcomes reported across studies, most notably for older age groups with acute hemorrhages [7]. Mortality rates have ranged from 40 to 90% for acute SDHs in select populations though more recent reports since 2010 have ranged from 15 to 67.2% for elderly patients [8,9,10,11,12]. Admittedly, there will always be some variation between studies, but such extreme differences have led to contradictory recommendations in the literature and underscore a greater issue at hand [10, 13,14,15]. In this investigation, we sought to elucidate upon the unique subpopulations being combined in the current conventional reporting of SDHs and proposed a stratification system to help standardize and improve reproducibility of future research on this topic.
Materials & methods
At a single, tertiary academic medical center, patients who underwent subdural hematoma evacuation between November 2013 and December 2021 were retrospectively identified using ICD 9 and 10 billing codes. Patients of any age with a SDH requiring surgical evacuation were included. In the event a single patient had multiple hospital admissions, only their first encounter was included. Initially 689 patients were captured, 195 of whom were excluded because of duplicate admissions, epidural hematoma evacuation, or no surgery transpired. In this retrospective, cohort study, data was retrieved from the electronic medical record included basic demographics, pertinent past medical history, method of arrival to the hospital, mechanism of injury, Glasgow coma scale (GCS) at time of arrival and discharge, length of stay (LOS), subdural hematoma size, laterality, presence of midline shift, surgery type, inpatient mortality, and neurological outcome at the time of discharge using the Glasgow Outcome Scale (GOS). Any GOS scores 1–3 were categorized as a “poor outcome.” In the ad hoc analysis, patients with a GCS score 8 or less at the time of arrival were categorized as having a “poor GCS score.” Subdural hematomas were classified as acute if the fluid collection was primarily hyperdense relative to the brain parenchyma and chronic if hypodense. Those with a mixed density or isodense were categorized as subacute. Surgery types were categorized as craniotomy, burr hole craniostomies, and craniectomy.
Mechanism of injury was reviewed by the authors and categorized as either positive or negative for a high-velocity impact (HVI) injury. Any head strike injury leading to the formation of a SDH that was incurred while traveling at a velocity beyond that of normal locomotion or daily activities was categorized as HVI. A fall from 10 feet or higher or motor-vehicle versus pedestrian was also counted as positive. Conversely, any assaults or a fall down a set of stairs were not. A list of presentations categorized as HVI are listed in Table 1. Using the proposed stratification system (Table 2), patients with an acute SDH were categorized by those with a HVI (aSDHHVI) in their history and those without (aSDHwo). Any patients with a subacute or chronic subdural hematoma were categorized as a mixed-SDH (mSDH).
Statistical analysis
Patient groups were compared using Student’s t-test for continuous parametric data. X2 was used for comparing categorical variables; if the expected frequency for an observation was less than 5 the Fisher exact test was used. One-way ANOVA test was used to compare the more than 2 continuous variables. Univariate logistic regression was used to compare the odds ratio of each proposed strata for the dependent variables “poor outcome” and “mortality”. A backwards stepwise multivariable logistic regression analysis was conducted in the acute SDH population with inclusion criteria set at p-value of less than 0.10 for the model. In an ad hoc analysis, the prognostic value of poor GCS score at time of arrival was compared to aSDHHVI by substituting aSDHHVI with poor GCS in the same multivariable logistic regression model. Since these two variables were not mutually independent, they could not be included in the same regression model without violating the statistical assumption for an absence of multicollinearity. The odds ratio for each respective variable and the model’s overall predictive accuracy using the area under the ROC curve were then compared. Only a p-value equal or less than 0.05 was considered significant and any missing observations were left blank during analysis. Statistical analysis was performed using STATA 14 (StataCorp LP, College Station, Texas). Approval from the hospital’s institutional review board to perform this clinical study and waiver of patient consent for data collection was obtained.
Results
A total of 494 patients with SDHs requiring evacuation were included in the study. The mean age was 68 years (Range 19 to 105) with a male predominance of 72%. The mean SDH size was 19 mm (Range 5–40 mm); midline shift was present in 81% of patients. The majority of SDHs were chronic (46%), followed by acute (32%), and subacute (22%). A total of 87 (18%) patients were on anticoagulation at the time of presentation. A detailed comparison of patient demographics, subdural hematoma characteristics, and co-morbidities by conventional SDH reporting is provided in Supplemental Table 1.
High-velocity impact and subdural hematomas
A total of 66 (13%) patients with clinical histories positive for HVI. Acute SDHs were stratified between those positive for a HVI history (aSDHHVI) to those without (aSDHwo) and those with mSDHs as shown in Table 3. aSDHHVI most often presented by ambulance (96% vs. 45% aSDHWO vs. 19% mSDH; p < 0.001), poor GCS 3–8 at arrival (52% vs. 21% aSDHWO vs. 1% mSDH; p < 0.001), longer LOS (31 days vs. 18 days aSDHWO vs. 10 days mSDH; p < 0.001), Trach/PEG placement (40% vs. 13% aSDHWO vs. 5% mSDH; p < 0.001), worse neurological status at discharge (43% vs. 25% aSDHWO vs. 8% mSDH; p < 0.001) and greater mortality (25% vs. 8% aSDHWO vs. 4% mSDH; p < 0.001). Patients with aSDHHVI exhibited 2.32x OR (95% CI 1.14–4.7, p = 0.02) for poor outcomes and 2.9x OR (95% CI 1.09–7.75, p = 0.03) for death compared to aSDHwo.
High-velocity impact had no association with poor outcomes (0% HVI group vs. 9% in w/o HVI group; p = 0.24) or death (0% HVI vs. 4% w/o HVI; p = 1) in patients with non-acute hemorrhages. Similarly, no difference in the symptom duration, mechanism of arrival, GCS scores on arrival or at discharge, or any other of the metrics were found between subacute and chronic SDH groups. A comparative distribution of poor outcomes between each group in the proposed stratification system is provided in Fig. 2. Next, the odds ratio for poor outcome and mortality was calculated for each strata using univariate logistic regression. The mSDH group was set as the baseline value for comparison. The results are listed in Fig. 3. All were significant with a p-value < 0.001 except for the mortality rate of aSDHwo compared to mSDH (OR 2.3, p = 0.061).
A distribution of poor neurological outcomes by the two different stratification systems is listed. Poor outcomes were categorized as Glasgow Outcome Scale 1–3. The upper bar represents the proposed stratification system. The light blue represent acute subdural hematoma patients with a high-velocity impact mechanism of injury (aSDHHVI). The dark blue block is acute subdural hematomas without HVI (aSDHwo). The beige colored block represents mixed-SDH (mSDH). The lower bar is the conventional reporting system with the light blue block representing acute subdural hematoma. The dark blue block represents subacute subdural hematoma. The beige block represents chronic subdural hematoma. A significantly greater rate of poor outcomes (p = 0.001) for patients with aSDHHVI than aSDHwo which would ordinarily be reported as a single group using the conventional system. Despite having these patients separated from the acute SDH population, the aSDHwo group still exhibited a significant difference in outcomes from those with mSDHs (p < 0.001) while none was found between subacute and chronic SDHs (p = 0.75)
Figure 3 depicts the results of the univariate logistic regression analysis for poor outcomes and mortality between each proposed SDH strata. The arrows represent the increasing odds ratio for each outcome using mSDH as the standard value. The red arrow on the left, which represents mortality rate, shows a 2.3 OR (95% CI 0.96–5.71, p = 0.06) for aSDHwo and 9.0 OR (95% CI 3.70-22.05, p < 0.001) for aSDHHVI patients. For poor outcome, aSDHwo had 3.4 OR (95% CI 1.9–6.2, p < 0.001) and aSHWHVI 11.5 (95% CI 5.64–23.3, p < 0.001). Abbreviations: SDH; subdural hematoma, w/o; without, w; with
Multivariable regression analysis in the acute SDH population
A backwards stepwise multivariable logistic regression analysis in the acute SDH population was performed with poor neurological status at the time of discharge as the dependent variable. Age at the time of surgery, gender, SDH size (mm), midline shift (mm), and HVI were initially evaluated. Only gender, midline shift (mm), anticoagulant use, and HVI met the inclusion criteria for the model. Female patients were less likely to experience poor outcomes than males (OR 0.34, 95% CI 0.15–0.75, p = 0.007) while midline shift (mm) (OR 1.08, 95% CI 1.00-1.17, p = 0.043), anticoagulant use (OR 3.24, CI 1.2–8.7, p = 0.019) and HVI (OR 6.85, CI 2.7–17.2, p < 0.001) were risk factors for poor outcomes. The AUROC demonstrated good predictive accuracy with a value of 0.74 and the Hosmer-Lemeshow goodness-of-fit test indicated a good fit (p-value 0.28).
Ad hoc analysis: glasgow coma scale versus high-velocity impact
An ad hoc analysis that compared outcomes using a GCS scoring system and the proposed HVI stratification system was conducted. Patients were categorized as having either a poor GCS score (≤ 8) or favorable GCS (> 8) in the aSDH population. A total of 47 patients comprised the poor GCS group while 110 were in the favorable group. There were 23 patients with cross-over between the poor GCS and aSDHHVI groups as they were not mutually exclusive. The rates of the aSDHHVI group versus the poor GCS group for each outcome metric are listed, respectively: arrival by ambulance (96% vs. 79%), mean length of stay (31 days vs. 27 days), trach/PEG placement (42% vs. 37%), poor outcomes (50% vs. 47%), reoperation rate (2% vs. 2%), and inpatient mortality (25% vs. 19%). Using the same multivariable logistic model as above (gender, midline shift (mm), and anticoagulation), HVI was substituted with poor GCS. Poor GCS had a odds ratio of 3.70 (95% CI 1.62–8.45, p = 0.002). The AUROC was 0.71 and Hosmer-Lemeshow goodness-of-fit test p-value of 0.89.
Discussion
As demonstrated in this study, SDH patients represent a highly heterogenous group. Despite possessing a range of presentation types and varying degrees of neurologic injury, SDH outcomes are often reported almost exclusively by the age of the blood products at the time of presentation. Although this method has its merits, this nomenclature has had unintended, adverse effects in the literature. Insufficient stratification of these subpopulation groups has contributed to conflicting conclusions across studies, making it is difficult to develop a consensus of opinion or treatment guidelines. This is particularly relevant for the advanced geriatric population with acute SDHs where a call for guidelines regarding the role for surgery and possible rationing of care has been made [9, 13]. With an anticipated surge in both the age and number of patients with SDHs requiring evacuation, the need for more accurate and reliable outcome reporting becomes greater each year [16].
Through incorporating a schematic that stratified groups by combining HVI and acute blood products, we were able to delineate upon two distinct patient populations that would have traditionally been shrouded under a single, acute SDH designation. For instance, those with aSDHHVI arrived at the hospital more often by ambulance (96% vs. 45%, p < 0.001), had a significantly longer hospital stay (31 vs. 18 days, p = 0.004), over double the rate of poor outcomes (50% vs. 23%, p = 0.001), and over triple the mortality (25% vs. 8%, p = 0.004) compared to the aSDHwo group. Outcomes of the aSDHwo also remained distinct from with mSDH group, demonstrating a difference in each metric listed above as well (Fig. 3). Among the acute SDH population, HVI alone carried over a 500% increased risk for poor outcomes (OR 6.85, p < 0.001) controlling for patient gender, midline shift, and anticoagulation use. The association of female gender, midline shift, and anticoagulation use corroborate prior studies that also have reported these as risk factors for poor outcomes [17,18,19].
Heterogeneity of Subdural Hematoma Outcome reporting
Until now, no prior studies have proposed a method to address the heterogeneity of SDH outcomes reported in the literature, to the best of our knowledge. A minority have attempted to stratify SDH outcomes based on traumatic injury type, presenting neurological condition, or GCS score [20,21,22,23]. While some SDH scoring systems exist, they have been aimed primarily at prognosticating and guiding treatment decision-making [24,25,26]. Consequently, there exists a need for a more specific and standardized categorization technique for SDHs beyond that of blood-age chronicity [27].
Surgical intervention of SDH has become controversial due to concerns for efficacy in the face of alarmingly high poor outcomes and mortality in the advanced geriatric population [13, 28]. Benedetto et al. (2017) reported a 6-month mortality rate of 67.2% in a series of 67 patients over 70 years with acute SDHs surgically evacuated [9]. Petridis et al. (2009) reported an inpatient mortality of 53.8% in a series of patients 65 or older [29]. For chronic SDHs, Whitehouse et al. (2016) reported 15 times the rate of poor outcomes of mortality for inpatient death for those 75 or older [30]. However, other studies reporting nearly half or less the rate of poor outcomes also exist around the same time period [15]. Won et al. (2017) found an inpatient mortality of only 28% in patients over 80 with aSDHs [10]. Younsi et al. (2021) reported an inpatient mortality of 33% for aSDHs [14]. Younger age groups have not been immune to these wide variations either. Lavrador et al. (2018) reported poor functional outcomes in 58% (GOS 1–3, 40/69) of those with aSDH while a series by Ryan et al. (2012) found 88% (184/210) of patients had good outcomes with a GCS score 13–15 at the time of discharge in the aSDH evacuated group [12, 31].
Causes of Subdural Hematoma Outcome Heterogeneity
Though it is difficult to say with absolute certainty, there are likely numerous factors contributing to these differences. A medical center’s surrounding population, location within a city, and the standards of practice can all be contributors. Relatively small sample sizes on this topic has also been cited as a potential cause [12]. In a large systematic review, Manivanne et al. (2021) cited differences in functional outcome metrics (i.e., GOS, GCS, Markslwalder scale, mRS) and follow-up time as possible contributors for heterogeneity of the pooled data from the literature [13].
An important factor at the crux of these differences is the proportion of patients with severe TBI and those with more isolated SDHs in the acute SDH population. As shown here, the proportion of patients with HVI aSDHs compared to those without has the potential to significantly affect outcomes. Albeit less often, SDHs can occur absent of a TBI event. Rapid acceleration-deacceleration movements with or without head strike can potentially still lead to shearing of bridging veins. Spontaneous SDHs can occur in patients on anticoagulation as well those with intracranial hypotension [32,33,34,35].
Among those who experience a TBI of extreme severity, the SDH is often only one of many sources of neurological injury. These may include diffuse axonal injury (DAI), brain contusions, cerebral edema, blood brain barrier disruption, dysautoregulation of cerebral blood flow, changes in cellular metabolism, and surge of neurotransmitter release at neurotoxic levels [36,37,38,39,40]. Prior efforts to stratify patients by neurological exam at the time of presentation or TBI severity but this is not well-standardized or commonly performed [22, 41].
Devising an effective stratification system
In devising a stratification system that incorporated this information, we found designating those with a HVI injury in the acute SDH population (Table 1) effective. It provided sufficient stratification between patient populations to realize differences spanning several aspects of patients’ hospital course. Though HVI’s also occurred in those with subacute and chronic SDHs, the presence of older blood products in it of itself selected for patients with less severe or without TBIs. This is supported by HVI demonstrating no statistical association with patient functional outcomes (p = 0.24) or mortality (p = 1) in the mSDH group. Additionally, no differences in presentation types, GCS on arrival, or other outcome metrics in those with subacute and chronic SDHs were found. With the exception of those in the chronic group having greater mean age (74 vs. 65 years, p = 0.01), these groups were very similar including the use of burr hole craniostomies versus craniotomies (20% chronic vs. 14% subacute, p = 0.12). Consequently, the merging of these blood ages as a single category avoided redundancy as it pertained to general disease course for those being surgically treated. This coincides and supports articles in the literature that have categorized both subacute and chronic SDHs together as “chronic” [27, 42, 43].
Another important feature in this design was keeping the stratification categories simple, with minimal group numbers. Defining a high-velocity mechanism of injury is still relatively intuitive to assign and easily retrievable in the history-of-present-illness. In maintaining a 3-tiered system that keeps blood age as a distinguishing feature, its adaptation is also a relatively minor transition from convention. Granted, there are likely other valuable categorizing features such as concussion grade, specific trauma event, or presenting GCS score. Yet these come at the expense of adding more categories and potentially requiring the retrieval of additional data points. Additional categories stand to lower the denominator across studies and could exacerbate the effect of exaggerated outcomes due to smaller sample sizes [44]. Lastly, concomitant injuries are often sustained with motor-vehicle accidents, pedestrian versus motor vehicle, and other high kinetic energy mechanism of trauma [45, 46]. By stratifying by HVI in the aSDH patient population, this likely better selects for those who sustained these other injuries rather than isolated GCS scores, which can also have a significant effect on outcomes [47].
In the ad hoc analysis, aSDHHVI demonstrated a greater odds ratio (OR 6.8 vs. 3.7) and predictive value for poor outcomes at discharge (AUROC 0.74 vs. 0.71) than poor GCS upon arrival. However, comparing these variables is challenging since they are not independent from one another, and interpretation may be limited. More commonly used statistical methods such as Chi2 or McNamer test cannot be used due to a failure to meet the required assumption for mutual independence [48, 49]. Although demonstrating a correlation with poor outcomes is not the salient metric for evaluating the utility of a stratification system (as listed for the reasons above), these findings do suggest aSDHHVI may provide a better representation of the underlying pathological changes seen in patients with different types of aSDHs. For instance, seizures, post-concussive symptoms, and SDH mass effect may have caused patients to present with a poor GCS score but still experience significant recovery following evacuation. Conversely, irreversible damage associated with DAI, brain contusions, and other sources of neuron injury that may have occurred after a HVI injury could have hindered as rapid or robust of a recovery.
Limitations
Study limitations include the retrospective study design. The generalizability of our institutional findings may be biased by the hospital location and referral pattern. Future investigation to further validate the utility of this proposed stratification system is needed. It would have been informative to compare the rates of DAI between the HVI and non-HVI patients with aSDHs, but this was not possible as MRIs were routinely performed in this patient population. Additionally, only surgical patients were included in this analysis. It is unclear the utility of this stratification system for non-surgical candidates and warrants further investigation.
Conclusion
Studies investigating subdural hematomas have historically reported outcomes by grouping patients based on the age of the blood products seen on imaging. Among the innumerable types of presentations that precede a SDH, this approach has insufficiently stratified the varied disease course in patients reported. In turn, this has likely been a major contributing factor in the heterogeneity of patient outcomes reported in the literature. A growing demand for more reliable reporting is needed in light of projected population trends and related public health implications. By categorizing those with aSDH who suffered a HVI to those who did not, and combining those with subacute and chronic SDHs, meaningful distinctions between patient populations based on presentations, hospital course, and outcomes were identified. The HVI designation alone was associated with a greater than 500% risk for poor outcomes at discharge in the acute SDH population. Compared to an alternative stratification method such as poor GCS (≤ 8) at the time of arrival, aSDHHVI revealed a greater odds ratio and predictive accuracy for poor outcomes using separate multivariable logistic regression models. This three-tiered system is easily adaptable from current convention and serves as an important step towards addressing the need for more improved consistency for outcome reporting on this topic.
Data availability
No datasets were generated or analysed during the current study.
References
Rao MG, Singh D, Vashista RK, Sharma SK (2016) Dating of Acute and Subacute Subdural Haemorrhage: a histo-pathological study. J Clin Diagn Res 10:Hc01–07. https://doi.org/10.7860/jcdr/2016/19783.8141
United Nations DoEaSA, Population Division (2019) World Population Prospects 2019: Methodology of the United Nations population estimates and projections (ST/ESA/SER. A/425)
Scotti G, Terbrugge K, Melançon D, Bélanger G (1977) Evaluation of the age of subdural hematomas by computerized tomography. J Neurosurg 47:311–315. https://doi.org/10.3171/jns.1977.47.3.0311
Bergström M, Ericson K, Levander B, Svendsen P (1977) Computed tomography of cranial subdural and epidural hematomas: variation of attenuation related to time and clinical events such as rebleeding. J Comput Assist Tomogr 1:449–455. https://doi.org/10.1097/00004728-197710000-00011
Karibe H, Hayashi T, Hirano T, Kameyama M, Nakagawa A, Tominaga T (2014) Surgical management of traumatic acute subdural hematoma in adults: a review. Neurol Med Chir (Tokyo) 54:887–894. https://doi.org/10.2176/nmc.cr.2014-0204
Ng SY, Lee AYW (2019) Traumatic brain injuries: pathophysiology and potential therapeutic targets. Front Cell Neurosci 13:528. https://doi.org/10.3389/fncel.2019.00528
Akbik OS, Starling RV, Gahramanov S, Zhu Y, Lewis J (2019) Mortality and functional outcome in surgically evacuated Acute Subdural Hematoma in Elderly patients. World Neurosurg 126:e1235–e1241. https://doi.org/10.1016/j.wneu.2019.02.234
Monsivais D, Choi HA, Kitagawa R, Franch M, Cai C (2018) A retrospective analysis of surgical outcomes for acute subdural hematoma in an elderly cohort. Interdisciplinary Neurosurg 14:130–134. https://doi.org/10.1016/j.inat.2018.07.010
Benedetto N, Gambacciani C, Montemurro N, Morganti R, Perrini P (2017) Surgical management of acute subdural haematomas in elderly: report of a single center experience. Br J Neurosurg 31:244–248. https://doi.org/10.1080/02688697.2016.1244249
Won SY, Dubinski D, Brawanski N et al (2017) Significant increase in acute subdural hematoma in octo- and nonagenarians: surgical treatment, functional outcome, and predictors in this patient cohort. Neurosurg Focus 43:E10. https://doi.org/10.3171/2017.7.Focus17417
Howard MA 3rd, Gross AS, Dacey RG Jr., Winn HR (1989) Acute subdural hematomas: an age-dependent clinical entity. J Neurosurg 71:858–863. https://doi.org/10.3171/jns.1989.71.6.0858
Ryan CG, Thompson RE, Temkin NR, Crane PK, Ellenbogen RG, Elmore JG (2012) Acute traumatic subdural hematoma: current mortality and functional outcomes in adult patients at a Level I trauma center. J Trauma Acute Care Surg 73:1348–1354. https://doi.org/10.1097/TA.0b013e31826fcb30
Manivannan S, Spencer R, Marei O et al (2021) Acute subdural haematoma in the elderly: to operate or not to operate? A systematic review and meta-analysis of outcomes following surgery. BMJ Open 11:e050786. https://doi.org/10.1136/bmjopen-2021-050786
Younsi A, Fischer J, Habel C et al (2021) Mortality and functional outcome after surgical evacuation of traumatic acute subdural hematomas in octa- and nonagenarians. Eur J Trauma Emerg Surg 47:1499–1510. https://doi.org/10.1007/s00068-020-01419-9
Taussky P, Hidalgo ET, Landolt H, Fandino J (2012) Age and salvageability: analysis of outcome of patients older than 65 years undergoing craniotomy for acute traumatic subdural hematoma. World Neurosurg 78:306–311. https://doi.org/10.1016/j.wneu.2011.10.030
Hsieh CH, Rau CS, Wu SC et al (2018) Risk factors contributing to higher Mortality Rates in Elderly patients with Acute traumatic subdural hematoma sustained in a fall: a cross-sectional analysis using registered Trauma Data. Int J Environ Res Public Health 15. https://doi.org/10.3390/ijerph15112426
McMillian WD, Rogers FB (2009) Management of prehospital antiplatelet and anticoagulant therapy in traumatic head injury: a review. J Trauma 66:942–950. https://doi.org/10.1097/TA.0b013e3181978e7b
Munivenkatappa A, Agrawal A, Shukla DP, Kumaraswamy D, Devi BI (2016) Traumatic brain injury: does gender influence outcomes? Int J Crit Illn Inj Sci 6:70–73. https://doi.org/10.4103/2229-5151.183024
Farace E, Alves WM (2000) Do women fare worse? A metaanalysis of gender differences in outcome after traumatic brain injury. Neurosurg Focus 8:e6. https://doi.org/10.3171/foc.2000.8.1.152
Koç RK, Akdemir H, Öktem IS, Meral M, Menkü A (1997) Acute subdural hematoma: outcome and outcome prediction. Neurosurg Rev 20:239–244. https://doi.org/10.1007/BF01105894
Ushewokunze S, Nannapaneni R, Gregson BA, Stobbart L, Chambers IR, Mendelow AD (2004) Elderly patients with severe head injury in coma from the outset–has anything changed? Br J Neurosurg 18:604–607. https://doi.org/10.1080/02688690400022763
Christopher E, Poon MTC, Glancz LJ et al (2019) Outcomes following surgery in subgroups of comatose and very elderly patients with chronic subdural hematoma. Neurosurg Rev 42:427–431. https://doi.org/10.1007/s10143-018-0979-4
Bocca LF, Lima JVF, Suriano IC, Cavalheiro S, Rodrigues TP (2021) Traumatic acute subdural hematoma and coma: retrospective cohort of surgically treated patients. Surg Neurol Int 12:424. https://doi.org/10.25259/sni_490_2021
Sharma R, Rocha E, Pasi M, Lee H, Patel A, Singhal AB (2020) Subdural hematoma: predictors of Outcome and a score to Guide Surgical decision-making. J Stroke Cerebrovasc Dis 29:105180. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105180
Stanišic M, Pripp AH (2017) A Reliable Grading System for Prediction of Chronic Subdural Hematoma Recurrence requiring Reoperation after initial Burr-Hole surgery. Neurosurgery 81:752–760. https://doi.org/10.1093/neuros/nyx090
Shen J, Xin W, Li Q, Gao Y, Zhang J (2019) A Grading System for the prediction of unilateral chronic subdural hematoma recurrence after initial single Burr Hole Evacuation. Risk Manag Healthc Policy 12:179–188. https://doi.org/10.2147/rmhp.S222144
Nisson PL, Francis JJ, Michel M, Goel K, Patil CG (2024) Extreme-aged patients (≥ 85 years) experience similar outcomes as younger geriatric patients following chronic subdural hematoma evacuation: a matched cohort study. GeroScience. https://doi.org/10.1007/s11357-024-01081-8
Shapey J, Glancz LJ, Brennan PM (2016) Chronic subdural haematoma in the Elderly: is it time for a New Paradigm in Management? Curr Geriatr Rep 5:71–77. https://doi.org/10.1007/s13670-016-0166-9
Petridis AK, Dörner L, Doukas A, Eifrig S, Barth H, Mehdorn M (2009) Acute subdural hematoma in the elderly; clinical and CT factors influencing the surgical treatment decision. Cent Eur Neurosurg 70:73–78. https://doi.org/10.1055/s-0029-1224096
Whitehouse KJ, Jeyaretna DS, Enki DG, Whitfield PC (2016) Head Injury in the Elderly: what are the outcomes of Neurosurgical Care? World Neurosurg 94:493–500. https://doi.org/10.1016/j.wneu.2016.07.057
Lavrador JP, Teixeira JC, Oliveira E, Simão D, Santos MM, Simas N (2018) Acute Subdural Hematoma Evacuation: predictive factors of Outcome. Asian J Neurosurg 13:565–571. https://doi.org/10.4103/ajns.AJNS_51_16
Yagi T, Suzuki T, Nagata Y, Naruse H, Nakagawa O (1996) [The cases of acute spontaneous subdural hematoma]. No Shinkei Geka 24:665–669
Akioka N, Fukuda O, Takaba M, Kameda H, Saito T, Endo S (2007) Clinical investigation of acute spontaneous subdural hematoma cases. J Stroke Cerebrovasc Dis 16:109–113. https://doi.org/10.1016/j.jstrokecerebrovasdis.2006.11.007
Garbossa D, Altieri R, Specchia FM et al (2014) Are acute subdural hematomas possible without head trauma? Asian J Neurosurg 9:218–222. https://doi.org/10.4103/1793-5482.146612
de Noronha RJ, Sharrack B, Hadjivassiliou M, Romanowski CA (2003) Subdural haematoma: a potentially serious consequence of spontaneous intracranial hypotension. J Neurol Neurosurg Psychiatry 74:752–755. https://doi.org/10.1136/jnnp.74.6.752
Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR (2017) Traumatic Brain Injury: current treatment strategies and future endeavors. Cell Transpl 26:1118–1130. https://doi.org/10.1177/0963689717714102
Li M, Sirko S (2018) Traumatic Brain Injury: at the crossroads of Neuropathology and Common Metabolic endocrinopathies. J Clin Med 7. https://doi.org/10.3390/jcm7030059
Armstead WM (2016) Cerebral blood Flow Autoregulation and Dysautoregulation. Anesthesiol Clin 34:465–477. https://doi.org/10.1016/j.anclin.2016.04.002
Johnson VE, Stewart W, Smith DH (2013) Axonal pathology in traumatic brain injury. Exp Neurol 246:35–43. https://doi.org/10.1016/j.expneurol.2012.01.013
Gruenbaum BF, Zlotnik A, Fleidervish I, Frenkel A, Boyko M (2022) Glutamate Neurotoxicity and Destruction of the blood-brain barrier: key pathways for the development of Neuropsychiatric consequences of TBI and their potential treatment strategies. Int J Mol Sci 23. https://doi.org/10.3390/ijms23179628
Leitgeb J, Mauritz W, Brazinova A et al (2012) Outcome after severe brain trauma due to acute subdural hematoma. J Neurosurg 117:324–333. https://doi.org/10.3171/2012.4.Jns111448
Miranda LB, Braxton E, Hobbs J, Quigley MR (2011) Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg 114:72–76. https://doi.org/10.3171/2010.8.Jns10298
Blaauw J, Jacobs B, Hertog HMD et al (2022) Mortality after chronic subdural hematoma is associated with frailty. Acta Neurochir (Wien) 164:3133–3141. https://doi.org/10.1007/s00701-022-05373-w
Biau DJ, Kernéis S, Porcher R (2008) Statistics in brief: the importance of sample size in the planning and interpretation of medical research. Clin Orthop Relat Res 466:2282–2288. https://doi.org/10.1007/s11999-008-0346-9
Khorasani-Zavareh D, Bigdeli M, Saadat S, Mohammadi R (2015) Kinetic energy management in road traffic injury prevention: a call for action. J Inj Violence Res 7:36–37. https://doi.org/10.5249/jivr.v7i1.458
Zheng J, Ouyang Y, Zhang K et al (2024) Early vs late fixation of extremity fractures among adults with traumatic brain Injury. JAMA Netw Open 7:e241556–e241556. https://doi.org/10.1001/jamanetworkopen.2024.1556
Leitgeb J, Mauritz W, Brazinova A, Majdan M, Wilbacher I (2013) Impact of concomitant injuries on outcomes after traumatic brain injury. Arch Orthop Trauma Surg 133:659–668. https://doi.org/10.1007/s00402-013-1710-0
Eliasziw M, Donner A (1991) Application of the McNemar test to non-independent matched pair data. Stat Med 10:1981–1991. https://doi.org/10.1002/sim.4780101211
Schober P, Vetter TR (2019) Chi-square tests in Medical Research. Anesth Analgesia 129:1193. https://doi.org/10.1213/ane.0000000000004410
Funding
There was no funding provided for this research.
Open access funding provided by SCELC, Statewide California Electronic Library Consortium
Author information
Authors and Affiliations
Contributions
Author Contributions: Peyton Nisson: Conceptualization, Data Curation, Formal Analysis, Investigation, Writing-Original draft, Writing-Review and Editing, Visualization, Project Administration. John Francis: Investigation, Data Curation, Writing-Review and Editing. Michelot Michel: Investigation, Data Curation, Writing-Review and Editing. Takuma Maeda: Writing-Review and Editing. Chirag Patil: Conceptualization, Writing-Review and Editing, and Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Approval from [blinded]’s institutional review board (STUDY00001750) to perform this clinical study and waiver of patient consent for data collection was obtained.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nisson, P.L., Francis, J., Michel, M. et al. A proposed stratification system to address the heterogeneity of Subdural Hematoma Outcome reporting in the literature. Neurosurg Rev 47, 207 (2024). https://doi.org/10.1007/s10143-024-02444-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-024-02444-7