Abstract
The appropriate surgical treatment strategy was based on the regions of tumor invasion. There is no classification to aid the surgeon in selection. A retrospective study of the clinical data of patients who underwent resection of thoracic dumbbell tumors at the Neurosurgery and Thoracic Surgery Department of Hospital between January 1, 2016, and December 31, 2021 was conducted. Patient data, images, and surgical outcome data were collected. The thoracic spine was divided into areas A, B, and C with respect to the line through the middle of the intervertebral foramen and the line of the costo-transverse joint lateral margin in the horizontal plane. Type I tumors were located in areas A or A and B, type II tumors were located in areas B or B and C, and type III tumors were located in areas A, B, and C. Fifty-five patients with thoracic dumbbell tumors were surgically treated (mean age, 43.1 years; 22 (40%) female). The patients with type I and III tumors underwent the posterior approach, type III tumors had more bleeding during the operation and longer operation times than type I. Among the patients with type II tumors who underwent video-assisted thoracic surgery and the posterior approach, the posterior group had more bleeding and a longer operation time than the others. The patients with type III tumors underwent the combined approach and the posterior approach; although there was no clear difference in the bleeding volume or operation time, the combined approach group had a lower incidence of complications. The new classification of different types of thoracic dumbbell tumors can simply and effectively guide the selection of surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dumbbell tumors are common, accounting for 13–17% of all spinal tumors, and thoracic dumbbell tumors (TDTs) account for 35% of all dumbbell tumors of the spine [1]. Among thoracic dumbbell tumors, 95% are benign, and approximately 90% are nerve sheath tumors, of which neurofibromas and schwannomas make up the vast majority [2, 3]. TDTs commonly arise from neurogenic elements within the spinal canal or spinal nerve and usually involve the nerve root and encroach on the dura, intervertebral foramen, and paraspinal and thoracic cavities [1, 4, 5]. At present, the primary treatment for dumbbell tumors is surgical removal [6].
A previous study indicated that the posterior approach is a standard surgical approach for excising tumors located in the intraspinal canal and intervertebral foramen [7, 8], thoracic surgery is appropriate for excising tumors located in the thoracic cavity [9], and combined surgery is suitable for removing large tumors [10]. However, the features of TDTs that involve the spinal canal or the thoracic cavity or even both increase the difficulty of selecting the type of TDT that is most suitable for removal by neurosurgeons or thoracic surgeons or which type of TDT can be removed by a combined approach.
However, various classifications of dumbbell tumors have been proposed to guide the selection of surgical strategies for dumbbell tumor resection [7, 11]. The most common classification method included Eden’s, Asazuma’s, and Tong Liu’s systems [7, 12]. Although Eden’s classification was the earliest system proposed and the most widely used, it is limited in guiding the selection of surgical resection. Asazuma’s study focused on cervical dumbbell tumors and neglected lumbar and thoracic spinal dumbbell tumors. Tong Liu proposed a standardized classification to guide the selection of the posterior and combined approaches, but there is no clear description of which types of TDTs are suitable for removal via thoracic surgery or whether one-stage or two-stage combined surgery is suitable.
Consequently, it was necessary to develop a new classification system for TDTs to guide the selection of the best surgical approach. The purposes of this retrospective study are to propose a new classification system and to evaluate the validity of the new classification system to assist surgeons in choosing an appropriate surgical approach.
Materials and methods
We retrospectively screened all patients who underwent resection of TDTs in the neurosurgery and thoracic surgery departments of the Hospital between January 1, 2016, and December 31, 2021. The patients’ clinical characteristics, imaging results and follow-up outcomes were analyzed. Data, including the surgical procedure duration, blood loss volume, postoperative drainage volume, hospitalization time, and complications (graded according to the Clavien-Dindo classification [13]), were collected. The local ethics board of our institutions approved this study, which was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki. Patient consent was obtained from all patients enrolled in this study. This is an observational study that complies with the STROBE statement.
Inclusion and exclusion criteria
Patients who underwent surgical resection of tumors invading two or more anatomical regions were considered for inclusion. Patients who had undergone a second surgical resection were excluded. Inclusion criteria: primary tumor; the exclusion criteria: patients with recurrent tumors; patients who were lost to follow up.
Statistical analysis
SPSS software version 25.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. A difference analysis was performed using the independent T test and Mann–Whitney U test. Normally distributed data are presented as the means and standard deviations. A P < 0.05 was considered statistically significant.
Surgery
Posterior approach
All posterior surgeries were performed by the neurosurgeons in this study. Under general anesthesia, the patient was positioned prone on bolsters. A posterior midline incision was made by neurosurgeons to allow dissection of the subcutaneous soft tissue and muscle and to expose the lamina of centrum, which required resecting the tumor. For extradural tumor invasion, a unitized high-speed drill and rongeur are used to partly excise the lamina, facet, transverse process, and even part of the ribs, until the periphery of the tumor can be observed by microscope. Then, subcapsular resection is performed to remove the tumor, which invaded the intercostal artery and pleura, to protect the artery and pleura from injury. The neurosurgeon carefully divides the capsular of the tumor from the artery and pleura. If it cannot be divided, part of the capsular of the tumor should be preserved. Finally, the neurosurgeon can carefully discern the tumor-bearing nerve and remove it.
In case of intraspinal-extraspinal dumbbell tumors or the extent of the tumor extends beyond the midline, total laminectomy has been performed to expose the spinal canal tumor. The extraspinal tumor also completely exposed by excise facet, transverse process and even part of the ribs thereafter. Under microscope assistance, the intraspinal component should be removed first. A T-shaped incision was made in the dura using 2 steps: the first step was to make a vertical incision along the dura to observe the boundary of tumor, the second was to make a horizontal incision along the nerve root to ensure adequate visibility for complete tumor excision. Then, the neurosurgeon strips the tumor from the spinal cord carefully. The tumor tissue is liberated, leading to gross total tumor excision. Finally, the dura was repaired primarily with running monofilament sutures and the T-shaped was covered with fibrin glue.
Video-assisted thoracic surgery
All video-assisted thoracic surgeries (VATSs) were performed by the thoracic surgeon involved in this study. The anesthesiologist performed pulmonary function evaluations in all the patients to identify whether the patients could tolerate collapse of the unilateral lung during the VATS. Patients were administered general anesthesia via a double-lumen endotracheal tube and positioned in a lateral decubitus position. The surgical incision was designed based on the location of the tumor. The ipsilateral lung was collapsed, and three 10-mm portals were made. One camera and two conventional instruments were placed into the thoracic cavity sequentially. The instruments were used to expose and remove the tumor. The tumor was placed in a disposable specimen bag. A chest tube was placed and the lung was reinflated under video guidance. Positive pressure was maintained in the pleura, and the wounds were closed.
Combined approach
All combined surgeries were performed by neurosurgeons who first removed the tumors in the spinal canal and then by thoracic surgeons who removed the tumors in the thoracic cavity by thoracotomy.
Classification
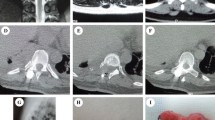
Based on the tumor location, degree of tumor spread, tumor characteristics, and anatomical characteristics of the thoracic vertebrae, we propose the classification of thoracic dumbbell tumors. We divided the thoracic vertebrae into three areas (A, B, C) in the horizontal position: area A was medial to the midline in the intervertebral foramen, area B was the area between the midline in the intervertebral foramen, and the vertical line through the costo-transverse joint lateral margin, and area C was lateral to the vertical line through the costo-transverse joint lateral margin. According to these areas, we formulated three types of dumbbell tumors: type I, the largest transverse sections of the tumor were located in area A (Fig. 1A) or both areas A and B (Fig. 1B); type II, the largest transverse sections of the tumor were located in area B (Fig. 2A) or both areas B and C (Fig. 2B); and type III, the largest transverse sections of the tumor were located in areas A, B, and C. In addition, depending on whether the dura was slit to access the intradural component, it was classified into two subtypes: type IIIA (Fig. 3A), in which the intradural dura was removed, and type III B (Fig. 3B), in which there was no need to open the dura.
Results
Fifty-five patients with TDTs underwent gross total resection of the tumor and the data are shown in Table 1. The baseline demographic data are presented in Table 2. The cohort included 22 female patients (41%), and the mean (SD) age was 43.1 (16.4) years. Of the 55 patients, 2 had a history of hypertension, 2 had a history of diabetes, and 1 had both. Of 55 operations, 29 (53%) were performed using the posterior approach, 23 (42%) were performed using VATS and 3 (5%) were performed using a combined approach (Table 3). The pathology of the tumor is presented in Table 4. Schwannoma was most common (45, 82%).
The clinical data of patients with type I and III tumors who underwent the posterior approach and were assessed during surgery are presented in Table 5. Ten type I and thirteen type III tumors were resected via the posterior approach. The patients with type I tumors had less intraoperative bleeding (202.0, 128.9, ml) and shorter operative times (163.5, 63.7, min) than those with type III tumors (480.7, 363.2, ml, 255.0, P = 0.03 75.7, min, P = 0.06). The patients with type III tumors had a higher incidence of complications; 6 patients (46%; I: 3; II: 2) who underwent the posterior approach experienced pleural effusion, 1 patient 1(7%; II: 1) experienced pneumothorax and 1 patient 1 (7%; II: 1) experienced pneumonia.
The clinical data of the patients with type II tumors who underwent the posterior approach and VATS were assessed during surgery to assess the surgical outcome are presented in Table 6. Twenty-three VATS procedures were performed and six posterior approach procedures were performed. The patients in the VATS group had less intraoperative bleeding (52.02, 35.1 ml) and shorter operative times (79.1, 35.1 min) than those in the posterior group (163.3, 112.5 ml, P = 0.02; 200.0, 34.2 min, P = 0.00). The patients in the two groups did not experience surgical complications.
The clinical data of the patients with type III tumors who underwent the posterior approach and combined approach and were assessed during surgery, and the surgical outcomes are presented in Table 7. Seven cases of type IIIA, including six cases, were treated by the posterior approach, and one case was treated by the combined approach. For the type IIIA patients, there were no distinct differences in the intraoperative bleeding volume (366.7, 175.1, ml; 400.0 ml, P = 0.29) and operative time (273.3, 175.1, min; 420.0 ml, P = 0.10) between the two groups. The patients in the two groups experienced several surgical complications. The combined approach resulted in several cases of cerebrospinal fluid leakage and pleural effusion. Four patients 4 (67%; I:3; II:1) who underwent the posterior approach experienced several pleural effusions, 1 patient (17%; II:1) experienced pneumothorax, and 1 patient 1 (17%; II:1) experienced a pneumonia.
Nine cases of type IIIB, including seven cases were treated via the posterior approach, and two cases were treated via the combined approach. There were also no distinct differences in intraoperative bleeding volume (578.6, 462.7, ml; 225.0, 35.4 ml, P = 0.33) or operative time (239.3, 84.8, min; 185.0, 7.1 ml, P = 0.42) between the two groups. The patients who underwent the posterior approach had surgical complications. One patient (14%; II:1) who underwent the posterior approach experienced several pleural effusions.
Among patients undergoing posterior surgery, the patients who underwent unilateral laminectomy and facetectomy received unilateral pedicle screw fixation, and those who underwent bilateral laminectomies and facetectomy underwent bilateral pedicle screw fixation.
Fifty-five patients were followed up in terms of clinical and radiographic outcome variables for 12 to 36 months (mean, 26 months). Fifty-three patients showed clinical improvement, but two patient showed no change in clinical status. There was no evidence of tumor recurrence or internal fixation failure in any of the patients.
Discussion
Type I tumor
The type I tumor originated from the spinal canal and did not extend beyond the vertical line through the costo-transverse joint lateral margin. In our 10 cases of type I tumors, all underwent the posterior approach. All patients underwent gross total resection and did not experience complications of surgery. The patients with type III tumors who underwent the posterior approach had more surgical bleeding, longer operative times and higher rates of complications than those with type I tumors.
The advantage of the posterior approach is the removal of tumors located in the spinal canal and intervertebral foramen region. For tumors located at the thoracic paravertebral or intrathoracic region, some surgeons utilize the L-shaped incision, C-shaped incision, or paraspinal approach to obtain vision while removing these tumors [7, 14].Although C- and L-shaped incisions have shorter lengths, amputated thoracic paraspinal muscles cause greater iatrogenic trauma, and the nonstraight incision has poor aesthetics and may hinder wound healing [15]. In addition, some surgeons have utilized the paraspinal approach to remove tumors [16, 17]. The approach has been used more frequently in lumbar spine surgery to reduce the risk of surgical injury. However, in the thoracic spine, the paraspinal muscles are not strong enough and do not have relevance compared with the lumbar spine. Thus, its advantage is nonsignificant compared to the lumbar spine. The approach makes it difficult to excise the tumor located at the spinal canal due to exposure [18, 19].
In our study, we observed that tumors that do not have a costotransverse joint lateral margin can utilize the posterior middle incision to resect tumors. Intraoperatively, the transverse costal joint was utilized as an anatomical marker to expose the tumor margin, and sufficient vision was obtained by rotating the surgical bed and microscope to cause less iatrogenic injury and reduce the risk of intercostal artery and pleural injury.
Type II tumor
Type II tumors were located outside the midline of the intervertebral foramen. VATS had obvious advantages over the posterior approach in terms of Type II tumors resection. The VATS group had less intraoperative bleeding and a shorter operative time but no significant difference in the length of hospital stays.
Some surgeons have indicated that the most noteworthy advantages of the posterior approach are that no chest drainage system is needed, which can shorten the hospitalization time. However, in our study, the patients who underwent the posterior approach did not show obvious advantages. There are clear advantages for the removal of type II tumors by VATS.
Type III tumor
Type III tumors arise from the spinal canal and involve the thoracic cavity more than the costo-transverse joint. Whether there was a component of the tumor in the dura mater was classified as type IIIIA or type IIIB. Although the combined approach did not show dominance in bleeding or duration of surgery, the patients who underwent the posterior approach had a higher incidence of pleural effusion.
The combined approach was characterized by changing the patient’s position during the operation and the cooperation of the two teams, leading to increased operation time and intraoperative bleeding. In fact, there was no significant difference between the posterior approach and the combined approach in bleeding volume or time. The combined approach indeed reduces the extent of resection of the facet, transverse process, and the rib and the stripping of the muscle from the spine compared with a single posterior approach.
At present, the general consensus regarding treatment for dumbbell tumors is that components of the intraspinal canal should be removed prior by the neurosurgeon to avoid overstretching the nerve [20,21,22]. However, whether the combined approach is a one- or two-stage resection has been debated. Compared to the two-stage approach, the one-stage approach is advocated because there is no risk of bleeding from remnant tumor tissue or compression of the spinal cord and hospital costs and the hospitalization time are reduced [21]. The advantages of the two-stage procedure, resection of the intraspinal and paravertebral components on separate occasions, are that both teams are free to operate within their own theatre environment with their own equipment on their own schedule [23]. More importantly, it conforms to the theory of damage control, reduced surgical trauma and accelerated postoperative recovery. For patients who have a weak physical condition and cannot tolerate lengthy surgery, two-stage surgery is likely a better surgical strategy.
Although we lack cases of the two-stage combined approach, the complications of severe pleural effusion and cerebrospinal fluid leakage in one case of a type IIIA tumor indicated that the type IIIA tumor was not suitable for the one-stage combined approach. Resection of type IIIA tumors includes intraspinal and extraspinal communication to the extraforaminal region, requiring that the dura be slit to remove the intraspinal component and the extraforaminal component be removed during thoracic surgery. Damage to the dura and pleura develops into a temporary pipeline, since the negative pressure of the chest leads to continuous cerebrospinal fluid leakage, leading to serious complications. Thus, we propose utilizing the two-stage combined approach to safely and effectively remove type IIIA tumors.
The resection of type IIIB tumors was performed by using a single posterior (n = 7) approach and a one-stage (n = 2) combined approach. Although the posterior approach had a higher incidence of complications than the combined approach, there was no significant difference in blood loss volume or operative time. Most of the TDTs were benign tumors. The neurosurgeons performed subcapsular resection to facilitate complete tumor removal. Thus, for the resection Type IIIB tumors, we suggest that the neurosurgeons attempt to completely remove the tumor. However, if it cannot be completely removed, the thoracic surgeon should attempt to remove the residual tumor.
Limitations
Our study has numerous limitations. First, the study was limited by its single-center, retrospective design. Second, the sample size was small because thoracic spinal dumbbell tumors are infrequently encountered. We did not have enough cases to be able to compare the advantages and disadvantages of the posterior approach with those of the combined approach in the resection of type III tumors. In addition, we still need to complete a prospective randomized controlled study to further illustrate the effectiveness and universality of the classification.
Conclusion
According to the classification we proposed, selecting an appropriate surgical strategy was indispensable for achieving safe, complete resection of the tumor while causing minimal trauma. The posterior approach was suitable for removing type I tumors. There is no doubt that a type II tumor can be excised using VATS. The two-stage approach is better for the removal of type IIIA tumors. For type IIIB tumors, we suggest that the neurosurgeons attempt to completely remove the tumor. If they cannot, the thoracic surgeon should attempt to resect the residual tumor (Fig. 4).
Data availability
The all date was included in the manuscripts.
References
Ozawa H, Kokubun S, Aizawa T, Hoshikawa T, Kawahara C (2007) Spinal dumbbell tumors: an analysis of a series of 118 cases. J Neurosurg Spine 7:587–593. https://doi.org/10.3171/spi-07/12/587
Ribet ME, Cardot GR (1994) Neurogenic tumors of the thorax. Ann Thorac Surg 58:1091–1095. https://doi.org/10.1016/0003-4975(94)90464-2
Barrenechea IJ, Fukumoto R, Lesser JB, Ewing DR, Connery CP, Perin NI (2006) Endoscopic resection of thoracic paravertebral and dumbbell tumors. Neurosurgery 59:1195–1201. https://doi.org/10.1227/01.Neu.0000245617.39850.C9. (discussion 1201-1192)
Ghostine S, Vaynman S, Schoeb JS, Cambron H, King WA, Samudrala S, Johnson JP (2012) Image-guided thoracoscopic resection of thoracic dumbbell nerve sheath tumors. Neurosurgery 70:461–467. https://doi.org/10.1227/NEU.0b013e318235ba96. (discussion 468)
Tomii M, Itoh Y, Numazawa S, Watanabe K (2013) Surgical consideration of cervical dumbbell tumors. Acta Neurochir (Wien) 155:1907–1910. https://doi.org/10.1007/s00701-013-1787-9
Agrawal A, Srivastava S, Joharapurkar SR, Gharde P, Ubeja G (2008) Single stage complete excision of large thoracic dumbbell schwannoma by modified posterior approach. Surg Neurol 70:432–436. https://doi.org/10.1016/j.surneu.2007.07.043
Liu T, Liu H, Zhang JN, Zhu T (2017) Surgical strategy for spinal dumbbell tumors: a new classification and surgical outcomes. Spine (Phila Pa 1976) 42:E748-e754. https://doi.org/10.1097/brs.0000000000001945
Konno S, Yabuki S, Kinoshita T, Kikuchi S (2001) Combined laminectomy and thoracoscopic resection of dumbbell-type thoracic cord tumor. Spine (Phila Pa 1976) 26:E130-134. https://doi.org/10.1097/00007632-200103150-00005
Liu HP, Yim AP, Wan J, Chen H, Wu YC, Liu YH, Lin PJ, Chang CH (2000) Thoracoscopic removal of intrathoracic neurogenic tumors: a combined Chinese experience. Ann Surg 232:187–190. https://doi.org/10.1097/00000658-200008000-00006
Chen X, Ma Q, Wang S, Zhang H, Huang D (2019) Surgical treatment of thoracic dumbbell tumors. Eur J Surg Oncol 45:851–856. https://doi.org/10.1016/j.ejso.2018.10.536
Jiang L, Lv Y, Liu XG, Ma QJ, Wei F, Dang GT, Liu ZJ (2009) Results of surgical treatment of cervical dumbbell tumors: surgical approach and development of an anatomic classification system. Spine (Phila Pa 1976) 34:1307–1314. https://doi.org/10.1097/BRS.0b013e3181a27a32
Asazuma T, Toyama Y, Maruiwa H, Fujimura Y, Hirabayashi K (2004) Surgical strategy for cervical dumbbell tumors based on a three-dimensional classification. Spine (Phila Pa 1976) 29:E10-14. https://doi.org/10.1097/01.Brs.0000103662.13689.76
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196. https://doi.org/10.1097/SLA.0b013e3181b13ca2
Rong HT, Fan YS, Li SP, Zhang ZS, Liu H, Liu T, Zhu T, Zhang JN (2018) Management of dumbbell and paraspinal tumors of the thoracic spine using a single-stage posterolateral approach: case series. Orthop Surg 10:343–349. https://doi.org/10.1111/os.12405
Gibson WP, Harrison HC (1997) Further experience with a straight, vertical incision for placement of cochlear implants. J Laryngol Otol 111:924–927. https://doi.org/10.1017/s0022215100138988
Wang R, Liang ZY, Chen Y, Chen CM (2022) Comparison of the clinical efficacy of transforaminal endoscopy and microtubular technology for the treatment of lumbar dumbbell-shaped tumors. Neurospine 19:513–523. https://doi.org/10.14245/ns.2244152.076
Li C, Ye Y, Gu Y, Dong J (2016) Minimally invasive resection of extradural dumbbell tumors of thoracic spine: surgical techniques and literature review. Eur Spine J 25:4108–4115. https://doi.org/10.1007/s00586-016-4677-z
Kalsi P, Zaidman N, Jain A, Casey ATH, Prezerakos G, Russo VM (2021) Surgical management of giant thoracic paraspinal schwannomas. World Neurosurg 149:e1155–e1165. https://doi.org/10.1016/j.wneu.2020.11.174
Ota M, Neo M, Fujibayashi S, Takemoto M, Nakamura T (2010) Advantages of the paraspinal muscle splitting approach in comparison with conventional midline approach for s1 pedicle screw placement. Spine (Phila Pa 1976) 35:E452-457. https://doi.org/10.1097/BRS.0b013e3181ce0696
Gossot D, Izquierdo RR, Girard P, Stern JB, Magdeleinat P (2007) Thoracoscopic resection of bulky intrathoracic benign lesions. Eur J Cardiothorac Surg 32:848–851. https://doi.org/10.1016/j.ejcts.2007.09.003
Cardillo G, Carleo F, Khalil MW, Carbone L, Treggiari S, Salvadori L, Petrella L, Martelli M (2008) Surgical treatment of benign neurogenic tumours of the mediastinum: a single institution report. Eur J Cardiothorac Surg 34:1210–1214. https://doi.org/10.1016/j.ejcts.2008.09.006
Ishikawa E, Matsumura A, Ishikawa S, Nakamura K, Nose T (2002) Combined minimally invasive approach using microsurgery and thoracoscopic surgery for resecting a dumbbell-type thoracic schwannoma. Minim Invasive Neurosurg 45:251–253. https://doi.org/10.1055/s-2002-36201
Harrison OJ, Bakir A, Chamberlain MH, Nader-Sepahi A, Amer KM (2021) Combined minimally invasive resection of thoracic neurogenic dumbbell tumors: a European case series. Thorac Cancer 12:2767–2772. https://doi.org/10.1111/1759-7714.14122
Author information
Authors and Affiliations
Contributions
M ZL and Z W wrote the main manuscript text, Z JA finished the data collection, L WX and Z W made the critical revision, W P and M ZL prepared the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was performed in line with the principles of the Guideline of Chinese GCP and ICH-GCP, and approved by the Institutional Review Board of Tangdu Hospital, Air Force Medical University (Date Nov 02, 2021/No. TDLL-202111–01).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zilong, M., Jinan, Z., Weixin, L. et al. Comparison of the surgical outcomes of the posterior approach, video-assisted thoracic surgery, and combined approach for thoracic dumbbell tumors based on a new classification: a retrospective study. Neurosurg Rev 47, 29 (2024). https://doi.org/10.1007/s10143-023-02267-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02267-y