Abstract
Craniocervical instability (CCI) is increasingly recognized in hereditary disorders of connective tissue and in some patients following suboccipital decompression for Chiari malformation (CMI) or low-lying cerebellar tonsils (LLCT). CCI is characterized by severe headache and neck pain, cervical medullary syndrome, lower cranial nerve deficits, myelopathy, and radiological metrics, for which occipital cervical fusion (OCF) has been advocated. We conducted a retrospective analysis of patients with CCI and Ehlers-Danlos syndrome (EDS) to determine whether the surgical outcomes supported the criteria by which patients were selected for OCF. Fifty-three consecutive subjects diagnosed with EDS, who presented with severe head and neck pain, lower cranial nerve deficits, cervical medullary syndrome, myelopathy, and radiologic findings of CCI, underwent open reduction, stabilization, and OCF. Thirty-two of these patients underwent suboccipital decompression for obstruction of cerebral spinal fluid flow. Questionnaire data and clinical findings were abstracted by a research nurse. Follow-up questionnaires were administered at 5–28 months (mean 15.1). The study group demonstrated significant improvement in headache and neck pain (p < 0.001), decreased use of pain medication (p < 0.0001), and improved Karnofsky Performance Status score (p < 0.001). Statistically significant improvement was also demonstrated for nausea, syncope (p < 0.001), speech difficulties, concentration, vertigo, dizziness, numbness, arm weakness, and fatigue (p = 0.001). The mental fatigue score and orthostatic grading score were improved (p < 0.01). There was no difference in pain improvement between patients with CMI/LLCT and those without. This outcomes analysis of patients with disabling CCI in the setting of EDS demonstrated significant benefits of OCF. The results support the reasonableness of the selection criteria for OCF. We advocate for a multi-center, prospective clinical trial of OCF in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The unique range of motion of the craniocervical junction relies upon the competence of ligaments joining the cranium to the upper two cervical vertebrae. Craniocervical instability (CCI) occurs in conditions of weakened ligaments such as trauma, infection, and connective tissue disorders. Inflammatory disorders, including rheumatoid arthritis and lupus, can also result in cranial settling and basilar invagination. Craniocervical instability and its phenotypic expression, the cervical medullary syndrome, have been increasingly recognized in conditions associated with ligamentous laxity. The latter include genetic conditions such as Down syndrome, congenital conditions such as Goldenhar syndrome, and hereditary disorders of connective tissue (HDCT), such as osteogenesis imperfecta, Marfan, Morquio, Stickler, and the Ehlers-Danlos syndromes [1,2,3,4,5]. Moreover, there is a recognized convergence of connective tissue disorders and “complex Chiari,” characterized by basilar invagination, kyphotic clival axial angle (CXA), and craniocervical instability [6,7,8,9,10]. This association has prompted increased consideration of dynamic imaging to better characterize the pathology and determine whether occipito-cervical fusion (OCF) may be indicated [11,12,13,14,15,16,17,18]. Emblematic of the HDCT are the 13 types of Ehlers-Danlos syndrome (EDS), characterized by weakness of connective tissue and many comorbid conditions, including neurological findings and dysautonomia attributed in part to chronic craniocervical and spinal instability [2, 19].
A growing body of literature suggests that chronic CCI manifests as a broad array of deleterious biomechanical effects upon the neural axis, in addition to causing altered cerebrospinal fluid and vascular flow [4, 8, 9, 12, 15,16,17, 20]. Headaches, long tract findings, motor delay and quadriparesis, dyspraxia, gait instability, and altered autonomic function are recognized as consequences of chronic biomechanical deformation of structures at the craniocervical junction in many hereditary connective tissue disorders [8, 13, 16, 19, 21,22,23,24,25]. There has been an evolving consensus in the literature of radiological metrics by which the presence, severity, and specific characteristics of CCI can be assessed and addressed [6, 8, 9, 13, 26,27,28,29,30,31,32,33,34,35,36].
Concurrent with this emerging understanding of chronic CCI is a need to validate clinical and radiological criteria by which individuals may be identified as appropriate candidates for OCF. This report describes a retrospective outcomes analysis of a cohort of patients with EDS and CCI who underwent OCF for severe, chronic, debilitating pain; symptoms of the cervical medullary syndrome; increasing neurological deficits; confirmatory radiological findings; and failed non-operative management. Our goal was to evaluate whether the surgical outcomes support the criteria by which patients were diagnosed with craniocervical instability and selected for OCF.
Methods
The study population consisted of a consecutive series of adults (n = 53) diagnosed with EDS [25] with clinical and radiological findings of CCI.
Perioperative data
All participants completed a clinical intake questionnaire on their initial visit, grading severity of pain, lightheadedness, syncope and presyncope, fatigue, mental clarity, and symptoms that constitute the cervical medullary syndrome [26]. Neurological examinations were performed by the neurosurgeons. Data were also extracted from clinical notes and other routine intake questionnaires.
Radiological findings of instability
Patients underwent dynamic MRI and CT imaging where possible. In some cases, flexion–extension X-rays were performed. Radiological measurements were performed by the neuroradiologist (MK).
-
1.
Dynamic upright flexion–extension cervical spine MRI
-
a.
Horizontal Harris Measurement (HHM)–the basion axis interval (BAI). Abnormal is ≥ 12 mm [13, 26, 30, 32, 34, 37] (Fig. 1).
MRI, cervical spine, mid-sagittal neutral view, T2 weighted (1.5 Tesla), showing a basion axis interval (white dashed line), measured from the posterior axial line (solid white line) to the basion. The BAI measures 15 mm. This exceeds the pathological threshold of 12 mm and constitutes radiological evidence of CCI
a Pathological translation of the basion with respect to the odontoid. Normally, between flexion and extension, the basion (b) pivots over the odontoid with < 2 mm of translation. In Fig. 2a, the cervical spine is in flexion, and the basion has translated anteriorly causing a bend in the brainstem. Note the BAI (*)—the interval measured from the basion to the posterior axial line (dashed line) is greater than the width of the spinal cord. b The cervical spine in extension shows straightening of the brainstem and a upper spinal cord and a shortened BAI. The change in BAI represents a pathological translation of the basion with respect to the spine. c MRI, upright, (dynamic), mid-sagittal, flexion view, T2 weighted (0.6 Tesla, Fonar Corp). The basion axis interval is 12 mm. d MRI, upright, (dynamic), mid-sagittal, extension view, T2 weighted (0.6 Tesla, Fonar Corp). The basion axis interval is 5 mm. Therefore, the BAI in flexion (12 mm) minus the BAI in extension (5 mm) represents a pathological translation of 7 mm
-
b.
BAI translation between flexion and extension (BAIflexion–BAIextension). Abnormal is ∆BAI > 4 mm [13, 23, 26, 29, 33, 34, 36, 38] (Fig. 2a, b, c, d).
-
c.
Clival axial angle (CXA). Abnormal is < 135° [9, 11, 26, 34, 39, 40].
-
d.
Ventral brainstem compression as measured by pBC2 measurement (also known as Grabb-Mapstone-Oakes (GMO) or Grabb-Oakes measurement). Abnormal is pBC2 ≥ 9 mm [27, 28, 34].
-
e.
MRI of cervical spine or brain to rule out Chiari malformation (CMI) (tonsillar herniation ≥ 5 mm), or low-lying cerebellar tonsils (LLCT) (tonsillar herniation < 5 mm), or foramen magnum (FM) stenosis [34, 41].
-
2.
Dynamic supine CT of the cervical spine with full neck rotation to left and to right to assess atlantoaxial instability (AAI) [8, 19, 29, 31, 32, 34, 38], measured by one of the following: C1C2 angular displacement ≥ 41°, or lateral displacement C1 upon C2 ≥ 4 mm on lateral head tilt, or > 80% loss of facet overlap on 3D CT reconstruction.
Indications for surgery
Patients undergoing surgery met each of these criteria (see Surgical Algorithm for the Treatment of Craniocervical Instability in the Ehlers Danlos Syndrome and Hypermobility Spectrum Disorder Populations Supplement):
-
1.
Severe head and/or neck pain (≥ 7/10 on the visual analog scale) for > 6 months.
-
2.
Symptoms of the cervical medullary syndrome: altered vision, diplopia, nystagmus, decreased hearing, dizziness, imbalance, vertigo, weakness, sensory loss, choking, dysarthria, dysphagia, sleep apnea or disordered sleep architecture, syncope, presyncope, and other dysautonomic symptoms [13, 19, 26, 42].
-
3.
Neurological deficits congruent with craniocervical instability such as lower cranial nerve deficits, weakness, sensory changes, hyperreflexia, Hoffman reflex, absent abdominal reflexes, Romberg sign, abnormal tandem gait, and dysdiadochokinesia.
-
4.
Failed non-operative management (neck brace, physical therapy, isometric exercises of the neck, activity modification, pain medication, and other modalities).
-
5.
Radiological findings of CCI as described above and one or more of the following additional radiological findings: (i) CMI, LLCT, or FM stenosis causing CSF flow obstruction; (ii) kyphotic clival axial angle; (iii) AAI; (iv) ventral brainstem compression (pBC2 ≥ 9 mm).
-
6.
Ability of the patient to understand the procedure, the risks and alternatives to surgery, and consent for surgery.
Exclusion criteria for surgery
Patients were excluded from surgery if less than 17 years of age, if they had undergone a previous craniocervical or atlantoaxial fusion, if they were pregnant, or if they were experiencing severe medical complications requiring ongoing treatment elsewhere.
The surgical procedure
Patients underwent open reduction/realignment and OCF [13, 14] at a single institution from 2018 to 2020 (Fig. 3a, b).
a CT scan, mid-sagittal view of the craniocervical junction showing the occipito-cervical fusion/stabilization (OCF). The bone allograft inserts superiorly into the aperture of the suboccipital plate. It lies against the occiput superiorly, the C1 posterior arch, and is notched inferiorly to encompass C2 spinous process and lamina. b CT scan, 3D reconstruction, showing the carefully tapered bone graft as it encompasses the occiput superiorly and lamina and C2 spinous process inferiorly. c The bone graft is secured within the aperture at the base of the CCI device (Cranio-Cervical Integration device, CCI®, LifeSpine Inc., Huntley, IL). The device has a smooth contour and small footprint to maximize bone surface area for fusion
To the extent possible, intraoperatively we brought the CXA into the normal range (> 140°) and eliminated ventral brainstem compression (pBC2 < 9 mm). We also established a normal or horizontal “gaze angle” (to avoid “star gazing”) and a mandibular axis interval (the measured interval from the anterior aspect of the C2 body to the posterior aspect of the mandible as seen on fluoroscopy or X-ray) > 10 mm and < 24 mm to avoid dysphagia [43]. Bone marrow, aspirated from the iliac crest, was injected into a saline-soaked, tricortical, iliac crest strip allograft for the fusion. The stabilization device used was the Solstice Cranio-Cervical Integration device (CCI®, LifeSpine Inc., Huntley, IL) (Fig. 3c).
The selected device presents a low, smooth profile, and a large aperture to incorporate a large bone graft. Postoperatively, patients were instructed to wear a neck brace for 1 month, and then to begin physical therapy.
Suboccipital decompression was performed for obstruction of CSF flow by Chiari malformation, low-lying cerebellar tonsils, or foramen magnum stenosis (AP diameter ≤ 30 mm). No durotomy was performed. The decompression included the full width of the foramen magnum, 20–25 mm to either side of midline, extending cephalad approximately 12 mm.
Preoperative data were collected from the questionnaires, the history, and neurological exam administered to every patient prior to surgery. Postoperatively, self-report questionnaires were emailed to participants. Additional data were collected from clinic records. Data were managed using Research Electronic Data Capture (REDCap), a secure, web-based software platform designed to support data capture for research studies. Questionnaires were completed by the patients postoperatively at 5–28 months (mean: 15.1 months).
Outcome measures
The primary outcome measures were as follows:
-
Severity and frequency of head and/or neck pain (both pre-op and post-op pain scores (1–5) as well as questionnaire on post-op improvement in pain severity/frequency. The pain scores were evaluated by comparing those patients with CSF flow obstruction from CMI, LLCT, or FM stenosis against those patients who did not have CMI or LLCT with CSF flow obstruction.
-
Use of pain medication
Secondary outcomes were as follows:
-
Changes in neurological, autonomic, and connective tissue disorder symptoms
-
Functional status (Karnofsky Performance Scale) [44]
-
Global Clinical Impression of Change score (changes in activity, symptoms, and quality of life since the surgery or last visit) [45]
-
Patient satisfaction survey
-
Orthostatic Grading Scale, in which patients reported the frequency and severity of orthostatic symptoms with daily activities before surgery and at final follow-up [46].
-
Wood Mental Fatigue Inventory. Before surgery and at the final follow-up, a subset of patients completed this 9-item questionnaire which asks how much in the preceding month the respondent was bothered by difficulty with memory, decision-making ability, concentration, processing, and symptoms of foggy head. Responses include 0 = not bothered at all, 1 = bothered a little, 2 = bothered somewhat, 3 = bothered quite a lot, and 4 = bothered very much; Possible scores ranged from 0 to 36 (maximal mental fatigue) [47, 48].
Data analysis
The primary analysis was a descriptive comparison of pre- and postoperative data for surgical patients. Statistical analyses were performed using Stata/IC software, version 15.1 (StataCorp, College Station, TX). Continuous variables are summarized as mean ± standard deviation or median (range), and categorical data were summarized as percentages. Chi-square, Fisher’s exact test, and Student t-test were used to analyze categorical and numeric data, respectively. The study was powered at 0.80 for the primary and secondary outcomes of interest, and a conservative two-tailed p value ≤ 0.01 was considered statistically significant for this descriptive study.
Results
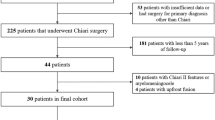
Fifty-three patients who had previous OCF met the criteria for inclusion in the study (Table 1).
Precipitating event prior to neurosurgery clinic visit
The mean time between onset of symptoms and neurosurgical evaluation was 12 years. Fifty percent of patients reported the onset of symptoms following a precipitating event; in the remainder, onset was gradual. The most common precipitating events were motor vehicle accidents (n = 7), pregnancy and childbirth (n = 2), sports injuries (n = 3), surgery (n = 3, including shoulder, spine, and median arcuate ligament surgery), and infection (n = 5).
Preoperative neurological deficits
The neurological exam was characterized in every patient by a combination of weakness, sensory deficits, loss of the gag reflex, dysdiadochokinesia, hyperreflexia, Romberg sign, Hoffman reflex, absence of abdominal reflex, and abnormal tandem gait. Apart from tussive headache, there were no signal findings that differentiated those patients with Chiari malformation or low-lying cerebellar tonsils from those with findings of instability alone.
Health care utilization and complications
All 53 patients had OCF and 32 patients also underwent suboccipital decompression in the same surgery. There were no intraoperative complications. Mean hospital length of stay was 4.3 days (SD, 1.2; range 2–8 days). Two to four weeks after surgery, four patients returned for re-operations for wound dehiscence. One of these four patients with a suspected infection returned for a revision of fusion 6 weeks later, after the cultures were negative. Within the follow-up period (average 15 months), 12 patients (23.1%) underwent surgeries for unrelated problems: tethered cord release (n = 12), sub-axial fusion (n = 3), placement of an intracranial pressure bolt (n = 1), and shunt (n = 2); and 12 patients (23.1%) were seen in the emergency room for issues not related to the surgery.
Primary outcomes: headache and/or neck pain and use of pain medication
Postoperatively, there was a significant improvement in headache and neck pain. Headache and neck pain both decreased from very severe, with a mean 4.3/5 pre-op, to moderate, with a mean 3.3/5 post-op (p < 0.001). Preoperatively, headache and neck pain scores for subjects with CMI/LLCT and CSF flow obstruction were the same as those with no CMI/LLCT and were similar postoperatively. There was no significant difference between the two groups (see Table 2).
Participants were also asked: “Has your head or neck pain changed in severity or frequency?” The patients overall reported significant improvement in terms of severity and frequency of head or neck pain (Fig. 4). There was no difference between the CMI/LLCT group and the non-CMI group of patients: improvement in the CMI/LLCT group was the same as improvement for the non-CMI group. Specifically, there was no significant difference of head and neck pain severity or frequency between the two groups (severity of headache p = 0.47; frequency of headache p = 0.30; neck pain severity p = 0.77, frequency of neck pain p = 0.92). Fifty-two percent of patients reported taking less pain medicine.
Secondary outcomes: improvement of Karnofsky Performance Status and symptoms
Karnofsky Performance Status (KPS) scores improved significantly from a preoperative median KPS = 50 (range 20–80) to postoperative median KPS = 60 (range 40–100) (p < 0.0001).
Postoperatively, there were significant improvements in the majority of neurological symptoms: nausea (p < 0.001), syncope (p < 0.001), presyncope (p < 0.001), speech difficulties (p = 0.002), concentration (p = 0.001), vertigo (p = 0.005) and dizziness (p = 0.001), photosensitivity and hyperacusis (p = 0.002), facial numbness (p = 0.002), arm weakness (p = 0.002), and incoordination (p = 0.001).
There was also significant improvement demonstrated for other important symptoms: fatigue (p = 0.001), palpitations (p = 0.002), muscle and joint pain (p = 0.001), chest pain at rest (p = 0.005), shortness of breath at night (p = 0.008), abdominal pain (p = 0.004), abdominal bloating (p = 0.013), pain in legs with ambulation (p = 0.013) (Table 3).
Patients reported improvement of orthostatic symptoms and mental fatigue
There was significant improvement in orthostatic symptoms in terms of the frequency, severity, types of activities of daily living, and standing time (p = 0.0006) (Fig. 5).
Thirty-two patients completed the Wood Mental Fatigue Inventory (WMFI) before surgery and at the last follow-up. Compared to prior to surgery, these patients reported significant improvement, with less confused or mixed-up thoughts, less difficulty making decisions, greater ability to listen while speaking, less “slow thoughts” and “foggy head” complaints, and less difficulty finding the right words (Table 4). The median WMFI score before surgery was 23 (0 being the best possible score with the least fatigue, and 36 being the maximal score of mental fatigue), and at the latest follow-up, it had improved to 18 (p = 0.005).
While this study was not designed to assess the impact of Chiari malformation on presenting features or outcome, the authors noted no signal differences in the outcomes of patients diagnosed with craniocervical instability and CMI/LLCT, as compared to those without CMI/LLCT.
Radiological findings
The radiological findings of CCI are presented (Table 5; N = 53).
Obstruction of CSF flow was assessed in 32/53 (60%) of patients, including 13 with LLCT, 18 Chiari malformation Type 1 (five of whom had previously undergone decompression), and 1 with FM stenosis. One additional patient with LLCT did not have CSF flow obstruction. The assessment of flow obstruction was based upon the limitation of CSF spaces imposed by the Chiari malformation, by a retroflexed odontoid [7], or by foramen magnum stenosis [41]. CSF flow studies were not performed before or after the fusion surgery. As a result of the intraoperative reduction, the preoperative kyphotic CXA (mean 128°) was brought into a normal range (mean postoperative CXA 142.8°; p < 0.0001), as measured at 3 months. The preoperative pBC2 (GMO measurement) (mean 9.1 mm) was brought into normal range (mean pBC2 = 6.18 mm; p < 0.0001).
Patient’s satisfaction with surgery and global impression of change
Participants reported a high level of satisfaction with the surgery, and 50/53 (94%) indicated they would repeat the surgery given the same circumstances; three (6%) indicated that they would not repeat the surgery. Forty of the 53 patients (75%) reported global improvement. Nine of the 53 patients (17%) reported worsening of their overall status due to co-morbid conditions, the most prominent of which were severe fatigue, mast cell activation syndrome, POTS, TMJ disorder, jugular vein compression with intracranial hypertension, low pressure syndrome due to presumed CSF leak, dystonia, and the need for further spinal surgery. Indeed, this group of patients reported a mean of 7 co-morbid conditions (Table 6). The Chiari malformation, after suboccipital decompression, was not considered to be a factor in postoperative disability in any of these patients.
Discussion
After failed non-operative management, 53 adult patients with severe head and neck pain, symptoms of the cervical medullary syndrome, congruent neurological deficits, and radiological findings of chronic instability of the craniocervical junction (CCI, AAI) underwent open reduction, stabilization, and OCF. Within this cohort of patients with CCI, Chiari Malformation 1 or CSF flow obstruction due to low-lying cerebellar tonsils or foramen magnum stenosis was frequently diagnosed (32/53) and treated with a limited foramen magnum decompression. This outcomes analysis is intended to assess the appropriateness of the indications and the efficacy of OCF in the treatment of instability in these patients (see Surgical Decision Algorithm Supplement). This series should be differentiated from, and not confused with, other series of CMI and basilar invagination [49]. Indeed, the authors concur that OCF is rarely indicated for CMI and should be reserved for patients in whom the primary underlying pathology is mechanical instability and those including the “complex Chiari” in whom significant deformity of the brainstem or upper spinal cord is manifest in the characteristic neurological presentation [13, 21, 28, 39, 40, 50].
Postoperatively patients reported improvement of pain and neurological symptoms
Postoperatively, most patients reported significant improvement in head and neck pain, in both severity and frequency, and there was a significant measured decrease in use of pain medication. At an average 15 months after surgery, when asked to compare their pain with the preoperative level, 13 patients reported minimal worsening and 3 reported much worsened neck pain. For head pain, 5 were minimally worse, and 2 were much worse. However, a review of the in-office questionnaires before and after surgery (Table 3) of these patients reporting worse pain (Fig. 4) showed that only one had reported increase in headache and only one an increase in neck pain score when compared to pre-op. This discrepancy shows the potential influences of recall bias over time as well as patients’ suffering from comorbid conditions. However, the authors recognize the opportunity to refine the selection criteria for surgery to improve pain outcomes.
The patients with CMI/LLCT were not differentiated from non-CM patients on the basis of pain. There was no significant difference in pain improvement between patients with CMI/LLCT and CSF flow obstruction compared to those without CMI/LLCT.
There was high patient satisfaction following surgery (94%). Patients reported significant objective improvement of syncope and presyncope and in the subjective symptoms of memory and concentration, weakness of the arms, dizziness, vertigo, nausea, speech difficulties, incoordination and balance, fatigue, palpitations, chest pain at rest or with activity, and leg pain while walking. Improvements were also demonstrated for diplopia, leg weakness, Raynaud’s phenomenon (fingers changing color with temperature), urinary frequency, and anxiety, though the latter did not reach statistical significance. The improvement of syncope and presyncope was mirrored in a significant improvement in the frequency and severity of orthostatic symptoms in most types of activities of daily living and in standing time (Fig. 5). Moreover, the improvement of memory, concentration, and fatigue was paralleled in the significant self-reported improvement in terms of the ability to make decisions, with less confused or mixed-up thoughts, greater ability to listen while speaking, less “slow thoughts” and “foggy head” complaints, and less difficulty finding the right words (Table 4).
Improvement of the symptoms of the cervical medullary syndrome (alternatively named the Cervico-cranial syndrome (ICD 10 code M53.0) or Brainstem Disability Symptoms is in keeping with the experience of others describing the treatment of basilar invagination, kyphotic CXA, CCI, and AAI due to incompetence of the craniocervical ligaments [3, 5, 9, 13,14,15,16,17, 22, 26,27,28, 34, 51].
The improvement of dysautonomia symptoms is attributed to mitigation of deformation of the sympathetic component of the autonomic nervous system [42, 52]. Ventral brainstem compression and instability result in chronic focal encephalopathy, affecting widely collateralized sympathetic neurons in the ventral lateral medulla, which project to preganglionic neurons at multiple spinal levels and also project to “generalist, bulbo-spinal, command neurons” in the central nervous system. The latter influence a broader network and provide tonic drive to cardiac and vascular structures [53, 54]. In this series, the authors attribute significant improvement of autonomic symptoms, in part, to the intraoperative open reduction, and restoration of a stable craniocervical junction with normal ventral brainstem contour.
Notwithstanding the significant improvements of subjective pain and symptoms, the Global Impression of Change found that only 75% of patients reported an improvement in overall quality of life, with 25% of patients reporting no improvement or worsening overall in the follow-up period. The latter must be seen in the context of the many co-morbid conditions from which EDS patients suffer. The legion of conditions (Table 6) included over 115 known diagnoses at the time of surgery of these patients. Commensurate with other reports of EDS patients [16, 19], a high number of patients had been previously treated or were subsequently treated by the authors, for tethered cord syndrome. The authors stress the importance of recognizing the presence of other medical issues, both before and after correction of the CCI, the importance of listening to the patients, and the need for referring them on for further diagnostic evaluation and treatment.
Radiologic metrics used to assess instability and brainstem deformity
CCI due to ligamentous instability is understandably more common in the populations with HDCT. Ligamentous laxity renders the craniocervical joints ill-equipped to maintain stability with multiaxial movements. Removal of posterior ligamentous and muscular structures in suboccipital decompression for Chiari malformation is associated with a high prevalence of iatrogenic CCI and kyphotic CXA [9, 11, 13,14,15, 26,27,28, 50]. The latter appears evident in the EDS population [13, 16, 19, 34, 40, 55].
While the clinical and radiographic algorithms for diagnosis and management of spinal instability in persons with EDS are evolving [56], it is generally recognized that CCI, basilar invagination, and ventral brainstem compression in these patients are often the result of ligamentous incompetence, and that these conditions require dynamic imaging for diagnosis. The authors note increasing acknowledgement of the metrics used in this study.[9, 10, 15, 19, 21, 27,29, 34, 40,49, 55,56,]. The BAI (aka, HHM) and BDI are useful and reliable measures of potentially pathological translation of the basion with respect to the odontoid. These measurements have the advantage that they do not require visualization of the opisthion or the posterior ring of C1, both of which structures are removed with prior suboccipital decompression (Fig. 1) [14, 19, 26, 29, 30, 32, 34, 40, 55, 56].
The mean BAI of 11 mm in our subjects is the same as reported by Marianayagam et al. (2021) among “complex Chiari” subjects who were shown to benefit from OCF [9]. Moreover, the basion-axis interval (BAI) may be measured on mid-sagittal views in flexion and extension to determine whether there is pathological translation [34]. Another important and more recent metric, the condylar-C2 sagittal vertical alignment (C-C2SVA), registers alignment and altered sagittal balance between the cranium (the atlanto-condylar joint) and the axis and is sensitive in the identification of the high-risk Chiari malformation patient that requires occipito-cervical reduction and OCF or ventral brainstem decompression [57].
Radiological evidence of craniocervical instability is not sufficient to diagnose pathological CCI
Radiological evidence of CCI does not in itself define clinically significant CCI. The authors rely on the doctrine of instability as a condition in which “the loss of the ability of the spine under physiological loads to maintain relationships between the vertebrae, in such a way that there is neither initial damage or subsequent irritation to the spinal cord or nerve roots, and in addition that there is no development of incapacitating deformity or pain due to the structural changes” [58]. Therefore, in the context of HDCT, pathological clinical instability requires the presence of neurological instability as evidenced by pain, symptoms, and deficits referable to the craniocervical junction, in addition to radiological evidence of instability. To be clear, the authors’ decision to consider OCF in patients with EDS was based primarily upon the severity of clinical findings and level of disability. CCI in the EDS populations is usually chronic, associated with a long history of increased pain with excessive motion, and must be diagnosed through the lens of a careful history and neurological examination.
In dealing with the population of patients with EDS, there remains difficulty in the determination of the point at which craniocervical hypermobility becomes CCI [56]. Populations of patients with more ligamentous laxity, such as children and persons with Down syndrome, generally exhibit up to 3 mm of basion-to-axis translation between flexion and extension due to ligamentous laxity [37]. In adults, hitherto, antero-posterior translation > 1 mm at CO/C1 was considered abnormal [13, 26, 29, 32, 34,35,36, 59]. A more recent retrospective radiology study of 50 adults undergoing upright dynamic MRI demonstrated a mean translation (∆ BAI) of 2.3 mm between flexion and extension. Notwithstanding that the patients of the latter study were imaged for neck pain and may therefore have had some inherent abnormality, the data argue for greater latitude of what constitutes normal translation [60]. We have used antero-posterior translation (∆ BAI) ≥ 4 mm as radiological evidence of instability [14] but acknowledge the need to establish normal parameters of basion-axis translation in patients without neck pain, especially in the population with HDCT.
The occipital-atlantal joint is normally a very stable “ball and socket” joint, which permits 10–20° of flexion extension, but less than 1 mm of translation and minimal rotation. This begs the question as to the basis of the pathological atlanto-occipital translation which we, and others, have described above [61]. A recent morphological study compared the occipital-atlantal joints of normal controls (n = 80) with patients with Chiari malformation and basilar invagination (n = 63). Detailed CT measurements of the occipito-atlantal joints demonstrated significantly smaller condyles and shallower superior facets of the C1 lateral mass in the patients with CMI and basilar invagination; the resulting dysplastic joints were permissive of excessive translation [62].
CCI also results from incompetence of both the condylar–C1 capsular lateral atlanto-occipital and the alar ligaments [7, 8, 13, 14, 40]. In our study, AAI (Fielding Type 1) was present in the majority of patients and was characterized by excessive rotational subluxation or lateral translation, loss of > 80% facet overlap, and decreased spinal canal diameter, but maintenance of a normal atlanto-dental interval [31, 32, 34, 38, 54, 55, 63, 64]. In many cases, the finding of AAI was an important factor in the decision to proceed with the OCF. The argument for AAI as a primary cause of cervical medullary syndrome has been made by Goel [7, 65].
The Park-Reeves consortium found the CXA for subjects needing OCF following posterior fossa decompression was significantly lower (128.8 ± 15.3°) than the subjects who did not require OCF [27]. We agree that correction of the kyphotic CXA (increasing or normalizing the CXA) is associated with improved clinical outcome [9, 13, 14, 21, 26, 27, 39, 50, 52].
Surgical technique and complications of craniocervical fusion
Open reduction allowed optimization of craniocervical relationships [43]. Suboccipital decompression was performed in 32 patients, in whom there was obstruction of CSF flow. The importance of unimpeded CSF flow through the foramen magnum has been emphasized [41].
Mao et al. noted the difficulty of occipital plate fixation after suboccipital decompression [56]. We were able to position a suboccipital plate following suboccipital craniectomy for Chiari malformation by using a low-profile system. There are many effective craniocervical systems available and many variations in technique, to accomplish the successful alignment and stabilization of the craniocervical junction [40, 66, 67]. We attribute the absence of intraoperative complications and injuries to the vertebral arteries to careful preoperative review of the CT and MRI imaging, precise entry points, angling of the C2 screws, and use of intraoperative fluoro-CT. The low complication rate in the present series is in keeping with others [3, 13, 14, 27, 28, 39, 40]. It is important to emphasize the 20% risk of a high or anomalous vertebral artery foramen, rendering screw placement dangerous [68]. In our series, shorter (16 mm) screws were occasionally placed in those cases where the vertebral artery foramen was very high and medial. Other techniques of stabilization, such as the occipital condylar screw fixation and the inside outside technique, have demonstrated an excellent record of safety and efficacy [40, 66, 67]. A low complication rate is evident where the OCF surgery is performed regularly, as evidenced by a study of 250 subjects undergoing OCF at one site in which 500 condylar screws were safely placed without screw pullout or vertebral artery impingement [66].
In our study, tricortical iliac crest strip allograft, infused with bone marrow aspirate, supplanted the use of rib autografts [13]. This avoided persistent pain from rib harvest and risk of exacerbating scoliosis. Pain overlying the suboccipital fixation devices, a frequent problem in a previous study, motivated the use in this study of a suboccipital plate with smooth contours, low profile, and small surface area [13]. While the wound dehiscence rate was disappointing, it is a recognized complication of EDS, in which slow wound healing and skin fragility are risk factors. Vicryl may incite inflammation in the epidermis, and consideration should be given to substitution with a non-inflammatory suture material, such as Prolene.
Legitimate concerns exist regarding increased adjacent segment degeneration and the need for further fusions at the subaxial levels [13]. The majority of these patients have significant premature degenerative disc disease and proclivity to subaxial instability [19]. The patient should be cognizant preoperatively of the possibility of needing further cervical fusion. In the authors’ opinion, however, this risk is mitigated by attention to posture and avoidance of injurious activity, especially neck flexion. Moreover, following OCF, the increased neuromuscular control of the neck and ability to exercise and strengthen the neck muscles may serve to improve neck stability.
There are concerns about loss of neck range of motion with OCF. It is the authors’ experience that patients very seldom complain of this, because of the increased range of motion conferred by the HDCT throughout the remainder of the cervical and upper thoracic segments. However, the absence of long-term follow-up of persons undergoing OCF should motivate the utmost care in the selection of patients who are suffering, who meet the indications for surgery, and who have failed a reasonable course of non-operative management.
Were the surgical indications appropriate?
The study suggests that utilization of the six criteria for OCF was associated postoperatively with statistically significant improvement in pain, mental fatigue, orthostatic and neurological symptoms, as well as non-neurological symptoms, such as fatigue and overall performance of daily activities, as shown by the improvement in KPS. As a retrospective analysis without a control group, this study does not validate the indications proposed for surgery. However, the outcomes analysis does support the reasonableness of the surgical criteria used in this study and demonstrates an association of these surgical criteria with favorable outcomes in the majority of cases. Moreover, these surgical criteria are concordant with others discussing OCF in the context of “Complex Chiari” or failed Chiari malformation surgery [3, 5, 9, 12,13,14,15,16,17, 27, 28, 34, 40].
Future directions
There remains a lack of consensus as to diagnostic imaging and management algorithms in the CCI and Chiari malformation literature [56, 69]. However, there is an increasing understanding of CCI as the manifestation of underlying ligamentous incompetence. Clearly, there is a need to standardize dynamic studies, to establish normative radiological interpretation of abnormal findings, and to aggregate data for the purpose of developing guidelines to determine which patients are most likely to benefit from surgery for CCI. The development of prospective, multi-center studies to validate the clinical indications and management is strongly recommended.
Conclusion
CCI is a well-described complication of patients with connective tissue disorders in general and the Ehlers-Danlos syndromes in particular. CCI is often recognized in failed suboccipital decompression for Chiari malformation and in “Complex Chiari.” Individuals with EDS who experience severe headache, neck pain, symptoms of the cervical medullary syndrome, neurological deficits, and radiological findings of CCI and who have failed non-operative management should be considered as potential candidates for OCF. Surgical intervention following utilization of these criteria is associated with significant improvement of pain, neurological symptoms, and disability following open reduction, stabilization, and OCF. However, there remains a need to understand long-term outcomes for this surgery. The many co-morbid conditions observed underscore the severe, multi-organ nature of EDS and the importance of understanding the multi-disciplinary care they require.
Availability of data and materials
Not applicable.
References
El-Khouri M, Mourão MA, Tobo A, Battistella LR, Herrero CFP, Riberto M (2014) Prevalence of atlanto-occipital and atlantoaxial instability in adults with Down syndrome. World Neurosurg 82:215–218. https://doi.org/10.1016/j.wneu.2014.02.006
Ellington M, Francomano CA (2023) Chiari I malformations and the heritable disorders of connective tissue. Neurosurg Clin N Am 34:61–65. https://doi.org/10.1016/j.nec.2022.09.001
Goel A, Bhatjiwale M, Desai K (1998) Basilar invagination: a study based on 190 surgically treated patients. J Neurosurg 88:962–968. https://doi.org/10.3171/jns.1998.88.6.0962
Henderson FC, Geddes JF, Crockard HA (1993) Neuropathology of the brainstem and spinal cord in end stage rheumatoid arthritis: implications for treatment. Ann Rheum Dis 52:629–637. https://doi.org/10.1136/ard.52.9.629
Menezes AH, Ryken TC, Brockmeyer DL (2001) Abnormality of the craniocervical junction. In: McLone DG (ed) Pediatric Neurosurgery. Surgery of the developing nervous system edn 4 Philadelphia, W.B. Saunders Co. pp 400–422
Dastagirzada YM, Kurland DB, Hankinson TC, Anderson RC (2023) Craniovertebral junction instability in the setting of Chiari malformation. Neurosurg Clin N Am 34:131–142. https://doi.org/10.1016/j.nec.2022.09.006
Goel A (2015) Is atlantoaxial instability the cause of Chiari malformation? Outcome analysis of 65 patients treated by atlantoaxial fixation. SPI 22:116–127. https://doi.org/10.3171/2014.10.SPINE14176
Loe ML, Vivas-Buitrago T, Domingo RA, Heemskerk J, Tripathi S, Bendok BR, Bydon M, Quinones-Hinojosa A, Abode-Iyamah K (2021) Prognostic significance of C1–C2 facet malalignment after surgical decompression in adult Chiari malformation type I: a pilot study based on the Chicago Chiari Outcome Scale. J Neurosurg Spine 34:171–177. https://doi.org/10.3171/2020.6.SPINE20544
Marianayagam NJ, Chae JK, Hussain I, Cruz A, Baaj AA, Härtl R, Greenfield JP (2021) Increase in clivo-axial angle is associated with clinical improvement in children undergoing occipitocervical fusion for complex Chiari malformation: patient series. J Neurosurg: Case Lessons 2:CASE21433. https://doi.org/10.3171/CASE21433
Ravindra VM, Brockmeyer DL (2023) Complex Chiari malformations: diagnosis, evaluation, and treatment. Neurosurg Clin N Am 34:143–150. https://doi.org/10.1016/j.nec.2022.08.009
Bollo RJ, Riva-Cambrin J, Brockmeyer MM, Brockmeyer DL (2012) Complex Chiari malformations in children: an analysis of preoperative risk factors for occipitocervical fusion: Clinical article. PED 10:134–141. https://doi.org/10.3171/2012.3.PEDS11340
Choi K-D, Choi J-H, Kim J-S, Kim HJ, Kim M-J, Lee T-H, Lee H, Moon IS, Oh HJ, Kim J-I (2013) Rotational vertebral artery occlusion: mechanisms and long-term outcome. Stroke 44:1817–1824. https://doi.org/10.1161/STROKEAHA.113.001219
Henderson FC, Francomano CA, Koby M, Tuchman K, Adcock J, Patel S (2019) Cervical medullary syndrome secondary to craniocervical instability and ventral brainstem compression in hereditary hypermobility connective tissue disorders: 5-year follow-up after craniocervical reduction, fusion, and stabilization. Neurosurg Rev 42:915–936. https://doi.org/10.1007/s10143-018-01070-4
Kim LJ, Rekate HL, Klopfenstein JD, Sonntag VKH (2004) Treatment of basilar invagination associated with Chiari I malformations in the pediatric population: cervical reduction and posterior occipitocervical fusion. J Neurosurg Pediatr 101:189–195. https://doi.org/10.3171/ped.2004.101.2.0189
Klekamp J (2012) Neurological deterioration after foramen magnum decompression for Chiari malformation Type I: old or new pathology?: Clinical article. PED 10:538–547. https://doi.org/10.3171/2012.9.PEDS12110
Milhorat TH, Bolognese PA, Nishikawa M, McDonnell NB, Francomano CA (2007) Syndrome of occipitoatlantoaxial hypermobility, cranial settling, and Chiari malformation Type I in patients with hereditary disorders of connective tissue. SPI 7:601–609. https://doi.org/10.3171/SPI-07/12/601
Nishikawa M, Ohata K, Baba M, Terakawa Y, Hara M (2004) Chiari I malformation associated with ventral compression and instability: one-stage posterior decompression and fusion with a new instrumentation technique. Neurosurgery 54:1430–1435. https://doi.org/10.1227/01.NEU.0000125326.15525.B8
Pindrik J, McAllister AS, Jones JY (2023) Imaging in Chiari I malformation. Neurosurg Clin N Am 34:67–79. https://doi.org/10.1016/j.nec.2022.08.006
Henderson FC, Austin C, Benzel E, Bolognese P, Ellenbogen R, Francomano CA, Ireton C, Klinge P, Koby M, Long D, Patel S, Singman EL, Voermans NC (2017) Neurological and spinal manifestations of the Ehlers-Danlos syndromes. Am J Med Genet 175:195–211. https://doi.org/10.1002/ajmg.c.31549
Rowe PC, Marden CL, Heinlein S, Edwards CC (2018) Improvement of severe myalgic encephalomyelitis/chronic fatigue syndrome symptoms following surgical treatment of cervical spinal stenosis. J Transl Med 16:21. https://doi.org/10.1186/s12967-018-1397-7
Dlouhy BJ, Menezes AH (2023) Management of ventral brainstem compression in Chiari malformation type I. Neurosurg Clin N Am 34:119–129. https://doi.org/10.1016/j.nec.2022.08.002
Celletti C, Galli M, Cimolin V, Castori M, Albertini G, Camerota F (2012) Relationship between fatigue and gait abnormality in joint hypermobility syndrome/Ehlers-Danlos syndrome hypermobility type. Res Dev Disabil 33:1914–1918. https://doi.org/10.1016/j.ridd.2012.06.018
Coban G, Coven I, Ciftci BE, Yildirim E, Yazici AC, Horasanli B (2013) The importance of craniovertebral and cervicomedullary angles in cervicogenic headache. Diagn Interv Radiol. https://doi.org/10.5152/dir.2013.13213
Klinge PM, McElroy A, Donahue JE, Brinker T, Gokaslan ZL, Beland MD (2021) Abnormal spinal cord motion at the craniocervical junction in hypermobile Ehlers-Danlos patients. J Neurosurg Spine 1–7. https://doi.org/10.3171/2020.10.SPINE201765
Malfait F, Francomano C et al (2017) The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet 175:8–26. https://doi.org/10.1002/ajmg.c.31552
Batzdorf U, Henderson FC, Rigamonte D et al (2015) Consensus Statement. In: Batzdorf U, Henderson FC, Rigamonte D (eds) CSF Colloquium on co-morbidities that complicate the treatment and outcomes of Chiari Malformation. Chiari and Syringomyelia Foundation Inc, NY, New York, pp 126–134
CreveCoeur TS, Yahanda AT et al (2021) Occipital-cervical fusion and ventral decompression in the surgical management of Chiari-1 Malformation and Syringomyelia: analysis of data from the Park-Reeves Syringomyelia Research Consortium. Neurosurg 88:332–341. https://doi.org/10.1093/neuros/nyaa460
Grabb PA, Mapstone TB, Oakes WJ (1999) Ventral brain stem compression in pediatric and young adult patients with Chiari I malformations. Neurosurgery 44:520–527; discussion 527–528. https://doi.org/10.1097/00006123-199903000-00050
Grahame R, Malik I, Hakim A, Koby M, Henderson F Sr (2020) Comment on “Quantitative measures of tissue mechanics to detect hypermobile Ehlers-Danlos syndrome and hypermobility syndrome disorders: a systematic review.” Clin Rheumatol 39(8):2481–2482
Harris JH, Carson GC, Wagner LK, Kerr N (1994) Radiologic diagnosis of traumatic occipitovertebral dissociation: 2. Comparison of three methods of detecting occipitovertebral relationships on lateral radiographs of supine subjects. Am J Roentgenol 162:887–892. https://doi.org/10.2214/ajr.162.4.8141013
Joaquim AF, Ghizoni E, Tedeschi H, Appenzeller S, Riew KD (2015) Radiological evaluation of cervical spine involvement in rheumatoid arthritis. Neurosurg Focus 38:E4. https://doi.org/10.3171/2015.1.FOCUS14664
Koby M (2016) The discordant report – pathological radiological findings: a peripatetic review of salient features of neuropathology in the setting of an erstwhile standard ‘normal’ radiological assessment. In: Batzdorf U (ed) Co-Morbidities that Complicate the Treatment and Outcomes of Chiari Malformation. Chiari Syringomyelia Foundation Inc., NY, NY, pp 50–52
Luciano MG, Batzdorf U, Kula RW, Rocque BG, Maher CO, Heiss J, Martin BA, Bolognese PA, Ashley-Koch A, Limbrick D, Poppe DJ, Esposito KM, Odenkirchen J, Esterlitz JR, Ala’i S, Joseph K, Feldman RS, Riddle R, Chiari I Malformation Common Data Element Working Group (2019) Development of common data elements for use in Chiari malformation type i clinical research: an NIH/NINDS project. Neurosurg 85:854–860.https://doi.org/10.1093/neuros/nyy475
Marathe N, Lohkamp L-N, Fehlings MG (2022) Spinal manifestations of Ehlers-Danlos syndrome: a scoping review. J Neurosurg Spine 37:783–793. https://doi.org/10.3171/2022.6.SPINE211011
White AA, Panjabi MM (1978) The clinical biomechanics of the occipitoatlantoaxial complex. Orthop Clin North Am 9:867–878
White AA 3rd, Panjabi MM (1990) The problem of clinical instability in the human spine: a systematic approach. In: Clinical Biomechanics of the Spine, 2nd edn. Lippincott, Philadelphia, p 285
Astin JH, Wilkerson CG, Dailey AT, Ellis BJ, Brockmeyer DL (2020) Finite element modeling to compare craniocervical motion in two age-matched pediatric patients without or with Down syndrome: implications for the role of bony geometry in craniocervical junction instability. J Neurosurg: Pediatrics 1–7. https://doi.org/10.3171/2020.6.PEDS20453
Riascos R, Bonfante E, Cotes C, Guirguis M, Hakimelahi R, West C (2015) Imaging of atlanto-occipital and atlantoaxial traumatic injuries: what the radiologist needs to know. Radiographics 35:2121–2134. https://doi.org/10.1148/rg.2015150035
Henderson FC, Henderson FC, Wilson WA, Mark AS, Koby M (2018) Utility of the clivo-axial angle in assessing brainstem deformity: pilot study and literature review. Neurosurg Rev 41:149–163. https://doi.org/10.1007/s10143-017-0830-3
Zhao DY, Rock MB, Sandhu FA (2022) Craniocervical stabilization after failed Chiari decompression: a case series of a population with high prevalence of Ehlers-Danlos syndrome. World Neurosurgery 161:e546–e552. https://doi.org/10.1016/j.wneu.2022.02.068
Heiss JD (2023) Cerebrospinal fluid hydrodynamics in Chiari I malformation and syringomyelia: modeling pathophysiology. Neurosurg Clin N Am 34:81–90. https://doi.org/10.1016/j.nec.2022.08.007
Petracek LS, Rowe PC (2023) Orthostatic intolerance and Chiari I malformation. Neurosurg Clin N Am 34:43–54. https://doi.org/10.1016/j.nec.2022.09.002
Henderson F Sr, Rosenbaum R, Narayanan M, Mackall J, Koby M (2020) Optimizing alignment parameters during craniocervical stabilization and fusion: A technical note. Cureus 12(3):e7160
Karnofsky D, Burchenal J (1949) The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod C (ed) Evaluation of chemotherapeutic agents. New York Press, Columbia University, pp 191–205
Hurst H, Bolton J (2004) Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther 27:26–35. https://doi.org/10.1016/j.jmpt.2003.11.003
Schrezenmaier C, Gehrking JA, Hines SM, Low PA, Benrud-Larson LM, Sandroni P (2005) Evaluation of orthostatic hypotension: relationship of a new self-report instrument to laboratory-based measures. Mayo Clin Proc 80:330–334. https://doi.org/10.4065/80.3.330
Bentall RP, Wood GC, Marrinan T, Deans C, Edwards RH (1993) A brief mental fatigue questionnaire. Br J Clin Psychol 32:375–379. https://doi.org/10.1111/j.2044-8260.1993.tb01070.x
Ross AJ, Medow MS, Rowe PC, Stewart JM (2013) What is brain fog? An evaluation of the symptom in postural tachycardia syndrome. Clin Auton Res 23:305–311. https://doi.org/10.1007/s10286-013-0212-z
Klekamp J (2022) Relevance of C1/2 facet configurations and clivus-canal-angles for adult patients with Chiari I malformation with and without Basilar invagination. World Neurosurg 162:e156–e167. https://doi.org/10.1016/j.wneu.2022.02.110
Kubota M, Yamauchi T, Saeki N, Yamaura A, Minami S, Nakata Y, Inoue M (2004) Surgical results of foramen magnum decompression for Chiari Type 1 malformation associated with syringomyelia: Spinal Surg 18:81–86. https://doi.org/10.2531/spinalsurg.18.81
Henderson F, Wilson W, Mott S, Mark A, Schmidt K, Berry J, Vaccaro A, Benzel E (2010) Deformative stress associated with an abnormal clivo-axial angle: a finite element analysis. Surg Neurol Int 1:30. https://doi.org/10.4103/2152-7806.66461
Henderson FC, Rowe PC, Narayanan M, Rosenbaum R, Koby M, Tuchman K, Francomano CA (2021) Refractory syncope and presyncope associated with atlantoaxial instability: preliminary evidence of improvement following surgical stabilization. World Neurosurg 149:e854–e865. https://doi.org/10.1016/j.wneu.2021.01.084
Farmer DGS, Pracejus N, Dempsey B, Turner A, Bokiniec P, Paton JFR, Pickering AE, Burguet J, Andrey P, Goodchild AK, McAllen RM, McMullan S (2019) On the presence and functional significance of sympathetic premotor neurons with collateralized spinal axons in the rat. J Physiol 597:3407–3423. https://doi.org/10.1113/JP277661
Geddes JF, Vowles GH, Hackshaw AK, Nickols CD, Scott IS, Whitwell HL (2001) Neuropathology of inflicted head injury in children. II. Microscopic brain injury in infants. Brain 124:1299–1306. https://doi.org/10.1093/brain/124.7.1299
Lohkamp L-N, Marathe N, Fehlings MG (2022) Craniocervical instability in Ehlers-Danlos syndrome—a systematic review of diagnostic and surgical treatment criteria. Global Spine J 12:1862–1871. https://doi.org/10.1177/21925682211068520
Mao G, Kopparapu S, Jin Y, Davidar AD, Hersh AM, Weber-Levine C, Theodore N (2022) Craniocervical instability in patients with Ehlers-Danlos syndrome: controversies in diagnosis and management. Spine J 22:1944–1952. https://doi.org/10.1016/j.spinee.2022.08.008
Ravindra VM, Iyer RR, Yahanda AT, Bollo RJ, Zhu H, Joyce E, Bethel-Anderson T, Meehan T, Smyth MD, Strahle JM, Park TS, Limbrick DD, Brockmeyer DL, Park-Reeves Syringomyelia Research Consortium (2021) A multicenter validation of the condylar-C2 sagittal vertical alignment in Chiari malformation type I: a study using the Park-Reeves Syringomyelia Research Consortium. J Neurosurg Pediatr 1–7. https://doi.org/10.3171/2020.12.PEDS20809
White AA, Johnson RM, Panjabi MM, Southwick WO (1975) Biomechanical analysis of clinical stability in the cervical spine. Clin Orthop Relat Res 85–96. https://doi.org/10.1097/00003086-197506000-00011
Werne S (1957) Studies in spontaneous atlas dislocation. Acta Orthop Scand Suppl 23:1–150
Nicholson LL, Rao PJ, Lee M, Wong TM, Cheng RHY, Chan C (2023) Reference values of four measures of craniocervical stability using upright dynamic magnetic resonance imaging. Radiol Med 128:330–339. https://doi.org/10.1007/s11547-023-01588-8
Zhou Q, Song C, Huang Q, Li H, Yang X, Peng L, Li J, Chen L, Shi L, Qi S, Lu Y (2022) Evaluating craniovertebral stability in Chiari malformation coexisting with type II Basilar Invagination: an observational study based on kinematic computed tomography and its clinical application. World Neurosurg 164:e724–e740. https://doi.org/10.1016/j.wneu.2022.05.045
Huang Q, Yang X, Zheng D, Zhou Q, Li H, Peng L, Ye J, Qi S, Lu Y (2023) Exploring the pathogenesis of atlanto-occipital instability in Chiari Malformation with type ii basilar invagination: a systematic morphological study. Neurosurgery 92:837–853. https://doi.org/10.1227/neu.0000000000002284
Fielding JW, Hawkins RJ (1977) Atlanto-axial rotatory fixation. (Fixed rotatory subluxation of the atlanto-axial joint). J Bone Joint Surg Am 59:37–44
Henderson FC, Rosenbaum R, Narayanan M, Koby M, Tuchman K, Rowe PC, Francomano C (2021) Atlanto-axial rotary instability (Fielding type 1): characteristic clinical and radiological findings, and treatment outcomes following alignment, fusion, and stabilization. Neurosurg Rev 44:1553–1568. https://doi.org/10.1007/s10143-020-01345-9
Fujiwara S, Tokunaga D, Oda R, Toyama S, Imai K, Doi A, Kubo T (2010) Dynamic close-mouth view radiograph method for the diagnosis of lateral dynamic instability of the atlantoaxial joint. Clin Imaging 34:375–378. https://doi.org/10.1016/j.clinimag.2009.08.027
Tam SKP, Bolognese PA, Kula RW, Brodbelt A, Foroughi M, Avshalumov M, Mugutso D, Ruhoy I (2022) Safety analysis and complications of condylar screws in a single-surgeon series of 250 occipitocervical fusions. Acta Neurochir 164:903–911. https://doi.org/10.1007/s00701-021-05039-z
Cunningham BW, Mueller KB, Mullinix KP, Sun X, Sandhu FA (2020) Biomechanical analysis of occipitocervical stabilization techniques: emphasis on integrity of osseous structures at the occipital implantation sites. J Neurosurg Spine 1–10. https://doi.org/10.3171/2020.1.SPINE191331
Wright NM, Lauryssen C (1998) Vertebral artery injury in C1–2 transarticular screw fixation: results of a survey of the AANS/CNS Section on Disorders of the Spine and Peripheral Nerves. J Neurosurg 88:634–640. https://doi.org/10.3171/jns.1998.88.4.0634
Bauer DF, Niazi T, Qaiser R, Infinger LK, Vachhrajani S, Ackerman LL, Jackson EM, Jernigan S, Maher CO, Pattisapu JV, Quinsey C, Raskin JS, Rocque BG, Silberstein H (2023) Congress of neurological surgeons systematic review and evidence-based guidelines for patients with chiari malformation: diagnosis. Neurosurgery 93(4):723–726. https://doi.org/10.1227/neu.0000000000002633
Acknowledgements
We are grateful to Dr. Robert Rosenbaum MD, FAANS, FACS for his significant contributions to the surgical technique for CCI and his participation in the surgeries reported herein. We also recognize the contributions of John Mackall in the development of the instrumentation and the use of allograft and harvested bone marrow for the fusion technique.
Funding
This work was supported by the Bruhn-Morris Family Foundation.
Author information
Authors and Affiliations
Contributions
F.C.H. Sr., J.R.S., M.L.N, K.T., S.E.M., M.B.K., P.C.R., and C.A.F. wrote the main manuscript text. K.T. and J.R.S prepared figures and performed statistical analyses. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the Institutional Review Boards at Greater Baltimore Medical Center and Indiana University School of Medicine.
Competing interests
Author F.C.H. Sr. sold intellectual property to Life Spine Inc. The other authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Henderson, F.C., Schubart, J.R., Narayanan, M.V. et al. Craniocervical instability in patients with Ehlers-Danlos syndromes: outcomes analysis following occipito-cervical fusion. Neurosurg Rev 47, 27 (2024). https://doi.org/10.1007/s10143-023-02249-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02249-0