Abstract
Objective
The aim of this study was to evaluate the effectiveness of ventriculo-cisternal irrigation (VCI) in preventing vasospasms and delayed cerebral infarction (DCI) by washing out subarachnoid clots earlier after aneurysm surgery.
Methods
We retrospectively identified 340 subarachnoid hemorrhage (SAH) patients with ruptured intracranial aneurysms treated with postoperative VCI at our institution between December 2010 and January 2020. As VCI therapy, a ventricular drain/cisternal drain was placed during aneurysm surgery, and lactated Ringer’s solution was used for irrigation until day 4 of SAH, followed by intracranial pressure control at 5–10 cmH2O until day 14.
Results
The median age was 65 years (interquartile range 52–75), with 236 female patients (69%). The World Federation of Neurosurgical Societies grade distribution was as follows: grade I or II, 175 patients (51%); grade III or IV, 84 (25%); and grade V, 81 (24%). With VCI management in all patients, total vasospasm occurred in 162 patients (48%), although the DCI incidence was low (23 patients [6.8%]). Major drainage-related complications were observed in five patients (1.5%). Early surgery, performed on SAH day 0 or 1, was identified as a preventive factor against DCI occurrence (odds ratio (OR) 0.21, 95% confidence interval (CI) 0.07–0.67; P = 0.008), while additional surgery (4.76, 1.62–13.98; P = 0.005) and dyslipidemia (3.27, 1.24–8.63; P = 0.017) were associated with DCI occurrence.
Conclusion
Managing vasospasms with VCI after SAH is considered a safe and effective method to prevent DCI. Early surgery after SAH may be associated with a decreased risk of DCI with VCI therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subarachnoid hemorrhage (SAH) is one of the most severe cerebrovascular diseases, with an incidence of 1–20 patients per 100,000 [24, 38]. Approximately 85% cases are caused by intracranial aneurysm rupture [24, 38]. Its morbidity and mortality remain high, and reruptured aneurysms lead to a mortality rate of approximately 50–60% [14, 21, 37]. Although these conditions often inevitably occur at SAH onset, appropriate interventions are required to prevent rerupture.
Cerebral vasospasm followed by delayed cerebral infarction (DCI) is the most difficult condition in acute to subacute phase of SAH, rarely resulting in severe neurological impairment. Reported incidence rates are up to 70% for angiographic vasospasm and 20–40% for DCI [2, 5,6,7, 9, 23, 25, 30]. However, mechanism of vasospasms and standard preventative or efficacious treatments remain unclear. Historically, predominantly in Japan, SAH has been addressed through postoperative interventions involving cisternal drainage for intracranial pressure (ICP) management and ventriculo-cisternal irrigation (VCI) therapy to facilitate the expulsion of intracranial clots; these initiatives have been associated with potentially enhanced outcomes through reduced occurrences of vasospasm and mitigation of the impact of DCI [13, 15, 17, 19, 27, 34]. In line with these attempts, we have treated many patients with aneurysmal SAH (including dissecting aneurysms) at our tertiary center and adopted VCI followed by ICP control as a unified method for over 20 years. This study aimed to present the outcomes of patients treated at our institution and evaluate the efficacy of VCI and ICP control in preventing vasospasms and DCIs.
Materials and methods
Patient selection
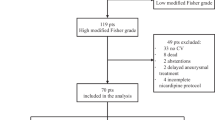
Between December 2010 and January 2020, we collected data for 373 patients who developed SAH due to ruptured cerebral aneurysms (including dissecting aneurysms) and underwent open surgery from an institutional SAH database. Thirty-three patients who did not undergo VCI therapy were excluded. VCI was not performed for the following reasons: (1) Fisher group 1 SAH with no cisternal clot to wash out (n = 5), (2) significant angiographical vasospasm in late phase surgery after onset (n = 9), (3) withdrawal from treatment immediately after surgery per the request of the patient’s family (n =18), and (4) inability to insert a ventricular drain (VD) in an appropriate position (n =1). Consequently, 340 patients who underwent surgery for SAH following VCI therapy and continuous ICP control were selected. Data regarding patient-, aneurysm-, SAH-, and surgery-related factors as well as clinical outcomes, which were prospectively collected and recorded in the database, were retrospectively evaluated. Informed consent was obtained from all participants, and the study was approved by the Institutional Ethics Committee of Showa General Hospital (approval number REC-328).
Postoperative SAH management
Figure 1A shows the treatment algorithm for aneurysmal SAH. When performing clipping or trapping surgery for SAH in Fisher group 2 or 3 with any cisternal clot, we performed VCI followed by 2 weeks of ICP control to prevent vasospasm, regardless of presence or absence of intraventricular hemorrhage. Preoperative assessment of cerebral arteries was basically performed by digital subtraction angiography (DSA); however, for patients requiring emergent surgery with increased intracranial pressure suspected, computed tomography (CT) angiography was used as an alternative. During surgery, we intraoperatively placed VD to manage cerebrospinal fluid (CSF) drainage and postoperative irrigation or drainage. VD was inserted via a frontal horn puncture in the case of surgery with supine position, or via a posterior horn puncture in the case of surgery with prone or park-bench position. Additionally, we placed a cisternal drain (CD) or lumbar drain (LD) for the CSF drainage system, which was used for postoperative management. CD was placed in the optico-carotid/precarotid cistern for the pterional approach or in the prechiasmatic cistern for the interhemispheric approach during surgery [13, 15, 17, 19, 27, 34]. In cases of surgery involving the posterior cranial fossa, LD was inserted instead of CD, postoperatively.
Schema showing our surgical treatment protocol and findings for a representative case. A Schema showing our surgical treatment protocol in the acute phase of aneurysmal subarachnoid hemorrhage (SAH), with ventriculo-cisternal irrigation (VCI) and continuous intracranial pressure (ICP) control therapy. Our approach to vasospasm prevention includes the following: (1) maximum washout of SAH by VCI until SAH day 4, (2) continuous cisternal drainage for ICP control during the vasospasm period after days 4 to 14, and (3) intensive care management and rehabilitation. B–E Representative case of SAH with a ruptured right middle cerebral artery aneurysm treated with our standardized method. B Preoperative computed tomography (CT) image. C, D On the day of SAH onset, aneurysm clipping is performed and ventricular (C) and cisternal drains (D; indicated by arrowheads) are placed. E After 4 days of VCI therapy, SAH has been completely washed out. CD cisternal drain, MRI magnetic resonance imaging, DSA digital subtraction angiography, OP operation
We administered a drop infusion system for irrigation with lactated Ringer’s solution, which was infused via VD at 20 mL/h and released from CD at the height of the forehead in the supine position. Starting on postoperative day 1, urokinase was added to the lactated Ringer’s solution at 120 U/mL to expedite clot removal through VCI. For patients with intraventricular hemorrhage, we initiated VCI inversely with infusion from CD and drainage from VD. On intraventricular hematoma resolution, we switched to regular circulation with infusion from VD and drainage from CD. After confirmation of SAH washout from CT images taken on day 4, we extracted VD and completed VCI. Thereafter, we continued CSF drainage with CD for ICP control around SAH day 14; CD was opened at the height of the forehead with the patient supine with a head-up position at 20–30°. Figure 1B–E shows representative images. During drain management, patients are generally kept at bed rest; however, for physically fit individuals, clamping drains during rehabilitation, meals, and bathroom breaks are implemented to ensure minimal disruption to activities of daily living. Cefazolin was administered intravenously during drainage as prophylaxis against meningitis.
Regarding imaging evaluation, we primarily assessed SAH distribution using CT scans until day 3. On day 4, magnetic resonance imaging (MRI) was performed to assess the early ischemic lesions and vascular conditions during the prevasospasm phase. On day 7, we performed DSA to evaluate the presence of cerebral vasospasm and aneurysms. We performed MRI to detect the presence of late-stage vasospasms and ischemic lesions on day 14. Until no evidence of clinical or angiographical vasospasms in MRI on day 14 was confirmed, close neurological examination was performed in intensive care unit. Once patients experience any neurological deficit, MRI or DSA was immediately performed to confirm status of brain and cerebral arteries.
Evaluated outcome
A detailed investigation on VCI therapy, including the route and duration, presence of urokinase, and ICP control period during VCI therapy was conducted. The primary outcomes were the vasospasm/DCI incidence and the DCI-induced symptoms. Vasospasm was defined as any angiographical vasospasm detected by DSA or magnetic resonance angiography (MRA) with a decrease of ≥ 50% in the cerebral artery diameter relative to the diameter on preoperative DSA or CT angiography. The judgement was based on radiological findings only, regardless of any symptoms [9]. DCI was defined as symptomatic infarction identified on CT or MRI after exclusion of procedure-related infarction, with any angiographical vasospasm [39]. Procedure-related infarction was excluded by MRI on day 4 or MRI within 2 days after the initial surgery in cases where the surgery was performed after day 4. All radiological findings were independently judged by two neurosurgeons. The secondary outcome was the prospectively recorded modified Rankin scale (mRS) score at discharge. A detailed safety assessment of the complications related to drainage management was also conducted. CSF infection, defined by a prolonged period of antibiotic therapy for control of systemic inflammation accompanied by an increase in white blood cells to > 500 counts/μL in CSF, was independently assessed.
Statistical analysis
The median and interquartile ranges (IQR) were calculated for each factor. Bivariate and multivariate logistic regression analyses were performed to determine the occurrence of vasospasms and DCI for each factor. The favorable outcome group at discharge was defined as an mRS score of 0–2, and the same analysis was conducted for this group. A P-value of < 0.05 was considered statistically significant. The factors used in the multivariate analysis were selected using a stepwise forward selection method with a P-value threshold of < 0.15. Statistical analyses were performed using JMP Pro 17 software (SAS Institute Inc., Cary, NC, USA).
Data availability
The authors confirm that data collected for the study and analysis methods will be shared upon reasonable request from any qualified investigator.
Results
Characteristics of patients, SAH, and surgeries
Table 1 shows the patient demographics and SAH factors. The median age was 65 years (IQR, 52–75 years), with female patients accounting for 236 patients (69%). Hypertension was the most common comorbidity and risk factor for arterial atherosclerosis. The World Federation of Neurosurgical Societies (WFNS) grades were as follows: grade I, 72 patients (21%); II, 103 (30%); III, eight (2%); IV, 76 (22%); and V, 81 (24%). Saccular aneurysms accounted for the majority, with 319 patients (94%), while 307 patients (90%) were treated for anterior circulation aneurysms. The most common aneurysm location was the internal carotid artery-posterior communicating artery, with 88 patients (26%), followed by the anterior communicating artery, with 86 patients (25%), and the middle cerebral artery, with 74 patients (22%). Preoperative DSA was performed in 316 patients and CT angiography was performed in 24 patients for angiographical assessment.
Surgical methods included aneurysmal neck clipping in 323 patients (95%) and parental artery trapping or proximal occlusion in 17 patients (5%) (Online Resource 1). Additional techniques included bypass in 23 patients (7%) and combined decompressive craniectomy in 22 patients (6%). Ventricular drainage before aneurysm treatment was performed in 44 patients (13%). The day of surgery for aneurysm treatment was day 0 (onset of SAH) in 193 patients (57%) and day 1 in 116 patients (34%), while treatment on day 2 or later was performed in 31 patients (9%). Early surgery on day 0 or 1 was performed more frequently in the WFNS grade IV/V group (98.1% vs. 84.7% in grades I–III group; P < 0.001). Additional surgery, including hematoma evacuation and decompressive craniectomy, was required in 31 patients (9%).
VCI and ICP control
VCI was performed using VD-CD irrigation in 268 patients (79%) and VD-LD irrigation in 72 patients (21%). Urokinase solution was used with VCI in 244 patients (72%). The median VCI duration was 5 days (IQR, 4–6 days), followed by a median duration of 26 days (IQR, 14–16 days) for ICP control with CD or LD (Online Resource 2).
Complications associated with drainage management were observed in 22 patients (6.5%), including five patients (1.5%) with severe complications resulting in permanent disability or requiring surgical intervention. The most common complication was bleeding around the ventricular drain in 10 patients (2.9%), two of whom (0.6%) required additional hematoma evacuation; the rest had minor bleeding without residual symptoms. Although intracranial hypotension during ICP management was observed in six patients (1.8%), only one (0.3%) required treatment, which involved hematoma evacuation for a surgical epidural hematoma. The patient fully recovered with a favorable outcome without any impairment (Online Resource 3). CSF infection was detected in 63 patients; however, no patient required prolonged hospitalization for this. Moreover, no patient developed a drainage-related abscess.
Cerebral vasospasm and delayed cerebral ischemia: incidence and risk analysis
Of the 340 patients, 162 (47%) exhibited total vasospasms (Table 2). Of these, 139 patients (41%) had transient vasospasms, and 23 (6.8%) eventually developed into DCI. Among the 23 patients with DCI, the most common symptom was hemiparalysis in 15 (4.4%), followed by disturbed consciousness in eight (2.3%), aphasia in seven (2.1%), other cognitive function impairments in five (1.5%), and sensory disturbances in two (0.6%). The group that underwent early surgery (days 0, 1) had a DCI incidence of 5.8%, whereas the group that underwent surgery after day 2 had an incidence of 16.1% (chi-square, P = 0.029).
Results of nominal logistic analysis of risk factors associated with total vasospasm and DCI occurrence are shown in Tables 3 and 4. In bivariate analysis, female sex, anterior circulation aneurysm, and the pterional approach were statistically significant risk factors for total vasospasm. In multivariate analysis, female sex (OR 1.64; 95% CI 1.01–2.67; P = 0.047) and anterior circulation aneurysm (OR 5.17; 95% CI 1.93–13.83; P = 0.001) remained statistically significant. Early surgery was a borderline factor for a significantly lower vasospasm risk (OR 0.47, 95% CI 0.22–1.04; P = 0.064). In bivariate analysis, risk factors related to DCI occurrence were dyslipidemia and postoperative additional surgery, while early surgery after SAH onset significantly reduced the risk of DCI. Multivariate analysis also identified dyslipidemia (OR 3.27; 95% CI 1.24–8.63; P = 0.017) and additional postoperative surgery (OR 4.76; 95% CI 1.62–13.98; P = 0.005) as statistically significant risk factors, with early surgery after SAH onset similarly reducing the DCI risk (OR 0.21; 95% CI 0.07–0.67; P = 0.008).
Overall clinical outcomes
At discharge, the performance status outcomes were as follows: favorable (mRS 0–2), 151 patients (44%); moderate (mRS 3, 4), 125 patients (37%); and severe (mRS 5, 6), 64 patients (19%) (Online Resource 4). The overall mortality rate was 5.3%. On stratification by WFNS grade, treatment for grades I and II achieved favorable outcome rates of 79% and 64% at discharge (Online Resource 5). In the grade IV and V groups, although 21% and 52%, respectively, ended up with severe outcomes (mRS 5, 6) at discharge, intermediate outcomes (mRS 3, 4) were observed for 55% and 41% patients while favorable outcomes were achieved for 24% and 7% patients, respectively (Online Resource 6). Exactly 113 patients (33%) required CSF shunt surgery because of secondary hydrocephalus, and 54 (16%) were discharged with a tracheostomy.
Bivariate and multivariate analyses of factors associated with favorable outcomes are shown in Table 5. In multivariate analysis, age ≥ 65 years (OR 0.11; 95% CI 0.06–0.22; P < 0.001), diabetes, anterior circulation aneurysm, concomitant ICH, WFNS grades 4 and 5, preoperative emergent VD, postoperative rerupture, and additional postoperative surgery were significantly negatively associated with a favorable outcome. Although early surgery was not associated with favorable outcomes in bivariate analysis, it was significant in multivariate analysis. Total vasospasm or DCI occurrence did not have a statistically significant negative impact on favorable outcomes at discharge.
Discussion
This is the largest cohort study to report the management of vasospasms after aneurysmal SAH through VCI and continuous ICP control therapy. Table 6 summarizes previous reports on VCI and ICP control [13, 15, 17, 19, 27, 34, 41]. Previous studies used CD for SAH washout after surgery, and VD was subsequently introduced, leading to VCI development. These reports indicated total vasospasm rates of 15.8–57.8% and symptomatic vasospasm rates of 2.8–39.5%, which align with the present findings [13, 15, 17, 19, 27, 34, 41]. DCI occurrence is generally reported to be 20–40% [2, 5,6,7, 9, 23, 25, 30], and reduced occurrence (0.9–20.0%) achieved with VCI and ICP control therapy, as observed in this study, is considered favorable [13, 17, 19, 34]. Particularly noteworthy is the excellent DCI occurrence rate of 0.9% achieved by Kodama et al., who used urokinase and ascorbic acid in the irrigation fluid; this requires further verification [19]. Furthermore, systematic reviews have shown the effectiveness of intrathecal nicardipine in reducing vasospasms and DCI [4, 10] and improving the proportion of patients with favorable outcomes (mRS 0–2) [33]. In light of the above, we explored the potential effectiveness of drainage management for ICP control to administer intrathecal nicardipine on radiological vasospasm occurrence. While few previous studies have described complications related to drainage management, Kodama et al. reported drain-related bleeding and ventriculitis, with a surgical requirement rate of 1.8%, but no residual sequelae [19]. We found a low rate of severe complications associated with drainage management (1.5%). With proficiency in the management of drainage systems, VCI and continuous ICP control can be performed safely after SAH surgery.
Early surgery was associated with a lower DCI risk; this result aligns with the clinical significance of VCI in removing subarachnoid clots as early as possible. Previous reports have also suggested the effectiveness of early surgery in reducing vasospasms and DCI, indicating the potential benefits of early aneurysm treatment combined with VCI and ICP control in reducing DCI [12, 36]. Furthermore, early surgery reportedly improved outcomes of SAH, and its usefulness has been incorporated into the guidelines [11, 16, 24, 26, 29]. Although no relationship between early surgery and favorable outcomes was observed, our findings suggest that patients requiring early surgery could be confounded by SAH severity. Patients with radiologically visible SAH treated with surgery in the early period after onset could be good candidates for VCI.
Our cohort included 22%/24% patients with WFNS grades IV/V, respectively, indicating a population with higher severity than that in previous reports on VCI [13, 17, 19, 34], which may explain the lower proportion of favorable outcomes (44%). While vasospasm or DCI occurrence was not associated with a favorable outcome, DCI did not show a significant positive effect on outcomes because of the low DCI incidence due to early surgery and management with VCI. We evaluated physical status at discharge using an assessment, potentially increasing favorable outcomes. Considering the above, compared with previously reported rates, favorable or intermediate outcomes, interpreted as outcomes where a bedridden status is prevented, were achieved in 48% patients, even in the WFNS grade V group [1, 18, 20, 31, 35, 40]. This finding could potentially imply the effectiveness of VCI therapy. Although less invasive treatment with stereotactic cisternal drainage has resulted in DCI prevention and favorable outcomes [32], in recent years, especially after clazosentan trials, discussions regarding vasospasm have progressed to revolve around a multifactorial etiology, including early brain injury, for worse outcomes [3, 8, 22]. Therefore, continuous ICP control through CD can result in improvements in prognosis. Further studies are needed to determine whether this management approach can improve the long-term performance status of patients. Against this background, a previous study showed that spreading depolarization, considered a cause of early brain injury and worse outcomes, can be triggered by spike-like increases in ICP [28]. Therefore, continuous ICP control through CD can result in improvements in prognosis. Further studies are needed to determine whether this management approach can improve the long-term performance status of patients.
Limitations
This study had several limitations. First, this was a single-center, single-arm, retrospective study without a comparative control group. Therefore, these results could not be single out VCI as an influencing factor for reduction of DCIs. In addition, this study is limited in its generalizability as it exclusively includes patients who underwent surgery for aneurysmal SAH, excluding those who underwent endovascular treatments. Second, only patients who received VCI treatment in this study were classified as Fisher grade 2 or 3, indicating a selection bias. Thus, the analysis may have been performed in a population more prone to vasospasms. Third, the clinical treatment outcome was assessed at discharge, and not all follow-up data after discharge were available. A long-term prospective comparable study is required to evaluate the effectiveness of this treatment.
Conclusion
VCI and continuous ICP control after surgery for SAH due to a ruptured aneurysm are considered effective and safe, reducing the DCI rate to 6.8%. Early surgery following SAH onset was particularly effective in preventing DCI.
Data availability
Available upon reasonable request.
References
Ariyada K, Ohida T, Shibahashi K, Hoda H, Hanakawa K, Murao M (2020) Long-term functional outcomes for World Federation of Neurosurgical Societies grade V aneurysmal subarachnoid hemorrhage after active treatment. Neurol Med Chir (Tokyo) 60:390–396. https://doi.org/10.2176/nmc.oa.2020-0052
Daou BJ, Koduri S, Thompson BG, Chaudhary N, Pandey AS (2019) Clinical and experimental aspects of aneurysmal subarachnoid hemorrhage. CNS Neurosci Ther 25:1096–1112. https://doi.org/10.1111/cns.13222
Demura M, Ishii H, Takarada-Iemata M, Kamide T, Yoshikawa A, Nakada M, Hori O (2023) Sympathetic nervous hyperactivity impairs microcirculation leading to early brain injury after subarachnoid hemorrhage. Stroke 54:1645–1655. https://doi.org/10.1161/STROKEAHA.123.042799
Dodson V, Majmundar N, El-Ghanem M, Amuluru K, Gupta G, Nuoman R, Wainwright J, Kaur G, Cole C, Santarelli J, Chandy D, Bowers C, Gandhi C, Al-Mufti F (2019) Intracranial administration of nicardipine after aneurysmal subarachnoid hemorrhage: a review of the literature. World Neurosurg 125:511–518e1. https://doi.org/10.1016/j.wneu.2019.01.103
Dorsch NW (1994) A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage part II: management. J Clin Neurosci 1:78–92. https://doi.org/10.1016/0967-5868(94)90080-9
Dorsch NW (1994) A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage part III: mechanisms of action of calcium antagonists. J Clin Neurosci 1:151–160. https://doi.org/10.1016/0967-5868(94)90021-3
Dorsch NW, King MT (1994) A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage part I: incidence and effects. J Clin Neurosci 1:19–26. https://doi.org/10.1016/0967-5868(94)90005-1
Endo H, Hagihara Y, Kimura N, Takizawa K, Niizuma K, Togo O, Tominaga T (2022) Effects of clazosentan on cerebral vasospasm-related morbidity and all-cause mortality after aneurysmal subarachnoid hemorrhage: two randomized phase 3 trials in Japanese patients. J Neurosurg 137:1707–1717. https://doi.org/10.3171/2022.2.JNS212914
Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, Connolly ES, Mayer SA (2009) Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke 40:1963–1968. https://doi.org/10.1161/STROKEAHA.108.544700
Hafeez S, Grandhi R (2019) Systematic review of intrathecal nicardipine for the treatment of cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Neurocrit Care 31:399–405. https://doi.org/10.1007/s12028-018-0659-9
Haley EC Jr, Kassell NF, Torner JC (1992) The international cooperative study on the timing of aneurysm surgery. The North American experience. Stroke 23:205–214. https://doi.org/10.1161/01.str.23.2.205
Inagawa T (1990) Effect of early operation on cerebral vasospasm. Surg Neurol 33:239–246. https://doi.org/10.1016/0090-3019(90)90042-n
Inagawa T, Kamiya K, Matsuda Y (1991) Effect of continuous cisternal drainage on cerebral vasospasm. Acta Neurochir 112:28–36. https://doi.org/10.1007/BF01402451
Inagawa T, Kamiya K, Ogasawara H, Yano T (1987) Rebleeding of ruptured intracranial aneurysms in the acute stage. Surg Neurol 28:93–99. https://doi.org/10.1016/0090-3019(87)90079-6
Ito U, Tomita H, Yamazaki S, Takada Y, Inaba Y (1986) Enhanced cisternal drainage and cerebral vasospasm in early aneurysm surgery. Acta Neurochir 80:18–23. https://doi.org/10.1007/BF01809552
Kassell NF, Torner JC, Jane JA, Haley EC Jr, Adams HP (1990) The international cooperative study on the timing of aneurysm surgery. Part 2: surgical results. J Neurosurg 73:37–47. https://doi.org/10.3171/jns.1990.73.1.0037
Kawakami Y, Shimamura Y (1987) Cisternal drainage after early operation of ruptured intracranial aneurysm. Neurosurgery 20:8–14. https://doi.org/10.1227/00006123-198701000-00003
Kobata H, Ikawa F, Sato A, Kato Y, Sano H (2023) Significance of pupillary findings in decision making and outcomes of world federation of neurological societies grade V subarachnoid hemorrhage. Neurosurgery 93:309–319. https://doi.org/10.1227/neu.0000000000002410
Kodama N, Sasaki T, Kawakami M, Sato M, Asari J (2000) Cisternal irrigation therapy with urokinase and ascorbic acid for prevention of vasospasm after aneurysmal subarachnoid hemorrhage. Outcome in 217 patients. Surg Neurol 53:110–117; discussion 117-118. https://doi.org/10.1016/s0090-3019(99)00183-4
Konczalla J, Seifert V, Beck J, Guresir E, Vatter H, Raabe A, Marquardt G (2018) Outcome after Hunt and Hess grade V subarachnoid hemorrhage: a comparison of pre-coiling era (1980-1995) versus post-ISAT era (2005-2014). J Neurosurg 128:100–110. https://doi.org/10.3171/2016.8.JNS161075
Lantigua H, Ortega-Gutierrez S, Schmidt JM, Lee K, Badjatia N, Agarwal S, Claassen J, Connolly ES, Mayer SA (2015) Subarachnoid hemorrhage: who dies, and why? Crit Care 19. https://doi.org/10.1186/s13054-015-1036-0
Lauzier DC, Jayaraman K, Yuan JY, Diwan D, Vellimana AK, Osbun JW, Chatterjee AR, Athiraman U, Dhar R, Zipfel GJ (2023) Early brain injury after subarachnoid hemorrhage: incidence and mechanisms. Stroke 54:1426–1440. https://doi.org/10.1161/STROKEAHA.122.040072
Macdonald RL, Pluta RM, Zhang JH (2007) Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol 3:256–263. https://doi.org/10.1038/ncpneuro0490
Mayberg MR, Batjer HH, Dacey R, Diringer M, Haley EC, Heros RC, Sternau LL, Torner J, Adams HP Jr, Feinberg W et al (1994) Guidelines for the management of aneurysmal subarachnoid hemorrhage. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 25:2315–2328. https://doi.org/10.1161/01.str.25.11.2315
Mijiti M, Mijiti P, Axier A, Amuti M, Guohua Z, Xiaojiang C, Kadeer K, Xixian W, Geng D, Maimaitili A (2016) Incidence and predictors of angiographic vasospasm, symptomatic vasospasm and cerebral infarction in chinese patients with aneurysmal subarachnoid hemorrhage. PLoS One 11:e0168657. https://doi.org/10.1371/journal.pone.0168657
Miyaoka M, Sato K, Ishii S (1993) A clinical study of the relationship of timing to outcome of surgery for ruptured cerebral aneurysms. A retrospective analysis of 1622 cases. J Neurosurg 79:373–378. https://doi.org/10.3171/jns.1993.79.3.0373
Ogura K, Hara M, Tosaki F, Hirai N (1988) Effect of cisternal drainage after early operation for ruptured intracranial aneurysms. Surg Neurol 30:441–444. https://doi.org/10.1016/0090-3019(88)90028-6
Oka F, Sadeghian H, Yaseen MA, Fu B, Kura S, Qin T, Sakadzic S, Sugimoto K, Inoue T, Ishihara H, Nomura S, Suzuki M, Ayata C (2022) Intracranial pressure spikes trigger spreading depolarizations. Brain 145:194–207. https://doi.org/10.1093/brain/awab256
Phillips TJ, Dowling RJ, Yan B, Laidlaw JD, Mitchell PJ (2011) Does treatment of ruptured intracranial aneurysms within 24 hours improve clinical outcome? Stroke 42:1936–1945. https://doi.org/10.1161/STROKEAHA.110.602888
Pluta RM, Hansen-Schwartz J, Dreier J, Vajkoczy P, Macdonald RL, Nishizawa S, Kasuya H, Wellman G, Keller E, Zauner A, Dorsch N, Clark J, Ono S, Kiris T, Leroux P, Zhang JH (2009) Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res 31:151–158. https://doi.org/10.1179/174313209X393564
Raabe A, Beck J, Goldberg J, Z’Graggen WJ, Branca M, Marbacher S, D’Alonzo D, Fandino J, Stienen MN, Neidert MC, Burkhardt JK, Regli L, Hlavica M, Seule M, Roethlisberger M, Guzman R, Zumofen DW, Maduri R, Daniel RT et al (2022) Herniation World Federation of Neurosurgical Societies scale improves prediction of outcome in patients with poor-grade aneurysmal subarachnoid hemorrhage. Stroke 53:2346–2351. https://doi.org/10.1161/STROKEAHA.121.036699
Roelz R, Schaefer JH, Scheiwe C, Sajonz B, Csok I, Steiert C, Buttler J, Rohr E, Grauvogel J, Shah MJ, Egger K, Niesen WD, Bardutzky J, Beck J, Coenen VA, Reinacher PC (2020) Impact of stereotactic ventriculocisternostomy on delayed cerebral infarction and outcome after subarachnoid hemorrhage. Stroke 51:431–439. https://doi.org/10.1161/STROKEAHA.119.027424
Sadan O, Waddel H, Moore R, Feng C, Mei Y, Pearce D, Kraft J, Pimentel C, Mathew S, Akbik F, Ameli P, Taylor A, Danyluk L, Martin KS, Garner K, Kolenda J, Pujari A, Asbury W, Jaja BNR et al (2022) Does intrathecal nicardipine for cerebral vasospasm following subarachnoid hemorrhage correlate with reduced delayed cerebral ischemia? A retrospective propensity score-based analysis. J Neurosurg 136:115–124. https://doi.org/10.3171/2020.12.JNS203673
Sakaki S, Ohta S, Kuwabara H, Shiraishi M (1987) The role of ventricular and cisternal drainage in the early operation for ruptured intracranial aneurysms. Acta Neurochir 88:87–94. https://doi.org/10.1007/BF01404143
Schwartz C, Pfefferkorn T, Ebrahimi C, Ottomeyer C, Fesl G, Bender A, Straube A, Pfister HW, Heck S, Tonn JC, Schichor C (2017) Long-term neurological outcome and quality of life after world federation of neurosurgical societies grades IV and V aneurysmal subarachnoid hemorrhage in an interdisciplinary treatment concept. Neurosurgery 80:967–974. https://doi.org/10.1093/neuros/nyw138
Solomon RA, Onesti ST, Klebanoff L (1991) Relationship between the timing of aneurysm surgery and the development of delayed cerebral ischemia. J Neurosurg 75:56–61. https://doi.org/10.3171/jns.1991.75.1.0056
Tanno Y, Homma M, Oinuma M, Kodama N, Ymamoto T (2007) Rebleeding from ruptured intracranial aneurysms in North Eastern Province of Japan. A cooperative study. J Neurol Sci 258:11–16. https://doi.org/10.1016/j.jns.2007.01.074
van Gijn J, Rinkel GJ (2001) Subarachnoid haemorrhage: diagnosis, causes and management. Brain 124:249–278. https://doi.org/10.1093/brain/124.2.249
Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, Terbrugge KG, Macdonald RL, Diringer MN, Broderick JP, Dreier JP, Roos YBWEM (2010) Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies proposal of a multidisciplinary research group. Stroke 41:2391–2395. https://doi.org/10.1161/Strokeaha.110.589275
Wostrack M, Sandow N, Vajkoczy P, Schatlo B, Bijlenga P, Schaller K, Kehl V, Harmening K, Ringel F, Ryang YM, Friedrich B, Stoffel M, Meyer B (2013) Subarachnoid haemorrhage WFNS grade V: is maximal treatment worthwhile? Acta Neurochir 155:579–586. https://doi.org/10.1007/s00701-013-1634-z
Yamamoto T, Mori K, Esaki T, Nakao Y, Tokugawa J, Watanabe M (2016) Preventive effect of continuous cisternal irrigation with magnesium sulfate solution on angiographic cerebral vasospasms associated with aneurysmal subarachnoid hemorrhages: a randomized controlled trial. J Neurosurg 124:18–26. https://doi.org/10.3171/2015.1.JNS142757
Funding
Open access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Contributions
M.U. made substantial contributions to the conception or design of the work, the acquisition, analysis, and interpretation of data; M.U. and G.Y. drafted the work or revised it critically for important intellectual content; M.U. and G.Y. approved the version to be published; and M.U. and G.Y. agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Ethics Committee of Showa General Hospital (approval number REC-328).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Umekawa, M., Yoshikawa, G. Impact of ventriculo-cisternal irrigation on prevention of delayed cerebral infarction in aneurysmal subarachnoid hemorrhage: a single-center retrospective study and literature review. Neurosurg Rev 47, 6 (2024). https://doi.org/10.1007/s10143-023-02241-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02241-8