Abstract
Objective cognitive function in patients with glioblastoma may depend on tumor location. Less is known about the potential impact of tumor location on cognitive function from the patients’ perspective. This study aimed to investigate the association between patient-reported cognitive function and the location of glioblastoma using voxel-based lesion-symptom mapping. Patient-reported cognitive function was assessed with the European Organisation for Research and Treatment (EORTC) QLQ-C30 cognitive function subscale preoperatively and 1 month postoperatively. Semi-automatic tumor segmentations from preoperative MRI images with the corresponding EORTC QLQ-C30 cognitive function score were registered to a standardized brain template. Student’s pooled-variance t-test was used to compare mean patient-reported cognitive function scores between those with and without tumors in each voxel. Both preoperative brain maps (n = 162) and postoperative maps of changes (n = 99) were developed. Glioblastomas around the superior part of the left lateral ventricle, the left lateral part of the thalamus, the left caudate nucleus, and a portion of the left internal capsule were significantly associated with reduced preoperative patient-reported cognitive function. However, no voxels were significantly associated with postoperative change in patient-reported cognitive function assessed 1 month postoperatively. There seems to be an anatomical relation between tumor location and patient-reported cognitive function before surgery, with the left hemisphere being the dominant from the patients’ perspective.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tumor location is an important factor to consider in surgical decision-making in glioblastoma patients, and a potential determinant of both preoperative objective cognitive impairment and perioperative changes [1,2,3,4]. The prevalence of cognitive deficits in glioblastoma patients ranges from 22 to 100% in different studies [5]. Structures involving language function, the basal ganglia, corpus callosum, cingulate cortex, and hippocampus are traditionally considered important for cognitive functions [6]. A range of advanced pre- and intraoperative tools may assist surgeons in identifying and minimizing the risk of damage to perceived eloquent regions [7,8,9]. However, which brain regions are the most important for cognitive function from the patients’ perspective is less known. A few previous studies have explored the relationship between patient-reported cognitive function and glioma location, but as a secondary outcome measure focusing on distinct and larger regions, such as lobes and lateralization [10,11,12,13]. Consequently, potentially important regions for patient-reported cognitive function may be overlooked [14]. In addition, since there is no consensus on how to define tumor location, it is often based on arbitrary criteria, which hampers objectivity and comparison of results. Voxel-based brain maps of three–dimensional (3D) segmented tumors can overcome some of these limitations and give a more accurate measure of tumor location.
In this population-based cohort study, we aimed to investigate the potential impact of tumor location on preoperative and postoperative change in patient-reported cognitive function in patients with glioblastoma using voxel–based lesion–symptom mapping (VLSM).
Materials and methods
Study population
Adult glioblastoma patients (≥ 18 years) scheduled for primary resection or biopsy only between September 2011 through December 2020 were eligible for inclusion. The patients were retrospectively identified from a regional brain tumor database at the Department of Neurosurgery, St. Olavs hospital, Trondheim, Norway. This department serves a defined geographical catchment region with approximately 750 000 inhabitants. The patients underwent surgery under general anesthesia, and the tumor was histopathologically classified as glioblastomas according to the 2007 or 2016 World Health Organization classification [15, 16]. Exclusion criteria were missing preoperative patient-reported cognitive function, known dementia, and/or missing preoperative magnetic resonance imaging (MRI) scans. Patients operated in several sequences (i.e., multifocal resections), undergoing biopsy only, and/or missing postoperative cognitive function score were included in the study of preoperative maps, but excluded from the maps of postoperative change.
Variables and data collection
Patient-reported cognitive function was reported by the patients themselves or with assistance from a nurse or family member 1–3 days before surgery and approximately one month after surgery (median 34 days, range 19–63 days) with the European Organisation for Research and Treatment (EORTC) QLQ-C30 cognitive function subscale (Norwegian translated) [17]. The EORTC questionnaire is a 30-items questionnaire that comprises a global quality of life scale, five functional scales, and six single items. The cognitive function subscale is one of the functional scales and includes the following two questions: During the past week: “have you had difficulty remembering things?” and “have you had difficulty in concentrating on things, like reading a newspaper or watching television?.” The questions are answered on a four-point scale from “not at all” to “very much.” The answers to these two questions were converted into a cognitive function score ranging from 0–100, with higher scores indicating better cognitive function [18].
The Karnofsky Performance Status (KPS) was scored prospectively by the operating surgeon [19]. In cases of missing KPS score (n = 3), medical notes were used to retrospectively estimate if the patients were functionally dependent (< 70) or independent (≥ 70). Patient- and treatment characteristics were retrieved from electronic medical records. Comorbidity was scored according to Charlson Comorbidity Index (CCI) [20], and postoperative complications within 30 days were graded according to the Landriel classifications system [21].
Brain imaging and segmentation
MRIs were routinely acquired < 72 h before surgery with a 1.5 or 3 Tesla MRI scanner. Tumor volumes were estimated by semi-automatic 3D tumor segmentation using the software packages 3D Slicer version 4.3.1–4.11 (3D Slicer, Boston, Massachusetts) [22] and BrainVoyager QX version 1.2 (Brain Innovation, Maastricht, Netherlands). We have previously demonstrated high agreement between these software packages [23]. In contrast-enhanced lesions, tumor volume was defined as pathological contrast enhancement and necrotic tissue within the contrast-enhancing borders seen on T1-weighted images (n = 156). In non-enhanced lesions, fluid attenuation inversion recovery images were used (n = 6). Images were segmented as part of several previous studies in glioblastoma. Several junior doctors/PhD students were trained in image interpretation by a neuroradiologist or an experienced glioma surgeon (OS) and image segmentations were reviewed by an neuroradiologist or the same neurosurgeon (OS). The extent of resection was calculated from pre- and postoperative MRI images as the relative postoperative reduction of the preoperative tumor volume in percentage and further dichotomized as gross total resection (100%) or subtotal resection (< 100%). In one patient, postoperative computed tomography (CT) images were used to determine subtotal extent of resection. Lateralization was categorized according to where the center of mass in each tumor was located, while multifocal bilateral tumors were categorized as a separate group.
Brain maps and statistical analyses
As described in a previous publication [24], the preoperative MRI segmentations were spatially aligned with the standardized frame of reference known as the Montreal Neurological Institute (MNI) space, defined by the ICBM-152 brain template [25]. Two sets of tumor maps were then created: one based on the preoperative EORTC cognitive function scores, and one based on the changes in function scores from preoperative to postoperative scoring. Each set consisted of three different maps: A distribution map showing the number of patients with a tumor in a given voxel, a statistical map showing voxels with a statistically significant correlation between the presence of a tumor and function score based on VLSM, and a descriptive map showing the mean cognitive function score for patients with tumor in a given voxel.
The VLSM analysis was performed using the NiiStat toolbox for Matlab (http://www.nitrc.org/projects/niistat). Here, for each voxel with at least three tumors, the patients were divided in two groups: those with tumor in the given voxel and those without. A Student's pooled-variance t-test was then performed to compare the cognitive scores between the two groups. The significance threshold was set to p ≤ 0.05. Then, since the test was performed for a large number of voxels, a permutation method [26] using 2000 permutations was applied to correct for multiple comparisons. Finally, the corrected threshold was applied to the map, retaining only voxels with statistically significant Z score.
SPSS Statistics version 28.0 (IBM, Armonk, New York) was used for descriptive statistics. Patient-, treatment- and disease characteristics are presented as either median with range or frequencies.
Results
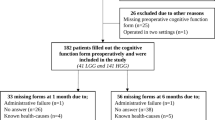
In total, 162 patients with glioblastoma were included in the preoperative brain maps (both resections and biopsies), and 99 patients were included in the postoperative change maps (resections only). The inclusion process is presented in Fig. 1.
Baseline and treatment characteristics
Baseline and postoperative treatment and disease characteristics are presented in Table 1. As seen, the median age was 62 years (range 29–83 years), and most patients were functionally independent with KPS ≥ 70 before surgery (86%). There were 74 glioblastomas (46%) located in the left hemisphere, 85 (52%) were right sided, while 3 (2%) were bilateral. There was no significant difference in preoperative tumor volume between the hemispheres (p = 0.14). Gross total resection was achieved in 31% of the patients, and 87% had initiated oncological treatment within one month of follow-up.
Brain maps with preoperative patient-reported cognitive function
Maps of preoperative tumor distribution, preoperative VLSM maps, and descriptive maps with mean preoperative patient-reported cognitive function, are presented in Fig. 2 and in Video 1 (Online Resource 1). The red spots in the VLSM maps show that several regions in the left deep central hemisphere were statistically significantly associated with preoperative patient-reported cognitive symptoms. The significant voxels were seen in the superior part of the left lateral ventricle, the lateral part of the left thalamus, the left caudate nucleus, and in a portion of the internal capsule just medial to the left arcuate fasciculus. Also based on the descriptive maps is seen that patients with tumors in the left hemisphere report worse preoperative cognitive function than patients with corresponding tumors in the right hemisphere. Particularly, tumor location in the left central structures, including the posterior part of the corpus callosum, the cingulate gyrus, hippocampus, and basal ganglia, seems to be linked to worse function, although not all regions were significant in the VLSM analyses.
Preoperative patient-reported cognitive function. The number under each cross section shows its coordinate in the ICBM-152 brain template [25]. A Number of tumors in each voxel, with darker blue indicating more tumors. B Voxels with a statistically significant correlation between the presence of tumor and cognitive function score (red) with atlas of arcuate fasciculus (yellow) and corticospinal tract (turquoise) for reference. The significance threshold of p ≤ 0.05 corresponded to a Z score < -4.91 after permutation correction. C Mean preoperative cognitive function in each voxel, with darker red indicating more cognitive problems
Brain maps with postoperative changes in patient-reported cognitive function
For patients who underwent surgical resection and with postoperative EORTC cognitive function score (n = 99), brain maps of preoperative tumor distribution and descriptive maps with mean change in patient-reported cognitive function at one month are presented in Fig. 3 and in Video 2 (Online Resource 2). The VLSM analysis of postoperative cognitive change was not significant, and hence no VLSM maps were created. From the descriptive maps, postoperative worsening of cognitive symptoms is seen in the left central hemisphere, including the posterior cingulate gyrus, and an area near the hippocampus. In contrast, patients harboring right hemisphere tumors more often reported unchanged or improved cognitive function.
Postoperative change in patient-reported cognitive function. The number under each cross section shows its coordinate in the ICBM-152 brain template [25]. A The ICBM-152 brain template for reference. B Number of tumors in each voxel, with darker blue indicating more tumors. C Mean postoperative change in cognitive function in each voxel, with green indicating improvement and red worsening
Discussion
This study used the VLSM method to examine the potential importance of tumor location for patient-reported cognitive function in a fairly large population-based sample of newly diagnosed glioblastoma patients. We found voxels in the left hemisphere to be significantly associated with worse preoperative cognitive function, including the lateral part of the left thalamus, the left caudate nucleus, and the left internal capsule, medial to the left arcuate fasciculus. Significant voxels were also found inside the left lateral ventricle, probably caused by inaccuracies in the registration of periventricular tumors due to mass effect and/or edema. Patient-reported cognitive function may be a relevant measure, and there seems to be an anatomical relationship between patient-reported cognitive function and tumor location.
Traditionally, the left hemisphere has been viewed as the most important and respected due to its dominance in language processing [27], and patients with left-hemispheric glioma are found to have more objective cognitive impairment than patients with right-sided glioma [2,3,4]. Our findings suggest that also from the patients’ perspective, the left hemisphere of the brain may be more dominant for cognitive function. We found an accumulation of significant voxels near the arcuate fasciculus, which connects the frontal, parietal, and temporal lobes and plays an important role in language processing [28]. Although language function was not formally tested in our study, language is presumed to play a central role in human cognition. It may therefore be difficult, also for the patients themselves, to separate language difficulties from other cognitive problems. Furthermore, we found an accumulation of significant voxels in the left internal capsule and the left lateral thalamus. Since the internal capsule contains fiber tracts coordinating cognitive pathways and the lateral thalamus relays limbic functions [29], more problems in these areas seem logical. Rather similar results are also found in other glioma studies [30, 31] and previous studies of objective cognitive function in stroke patients [32, 33]. Significant voxels were also seen in the caudate nucleus, which has a role in memory, learning, and executive functioning [34]. Another explanation for the left hemisphere's dominance may be that the right hemisphere plays a special role in cognitive functions that may not necessarily be picked up in the EORTC questionnaire, such as processing nonverbal information and perceptions of the body in relationship to the trunk and surrounding space [6].
Hemispheric distinctions in affective and emotional responses to brain damage may also explain the differences in patient-reported cognitive function between left-sided and right-sided tumors. Catastrophic reactions and stronger emotional responses to illness, as well as depression and anxiety, appear to be more frequent in patients with left hemispheric injury [35, 36]. Although many glioma studies have found no association between depression and hemispheric laterality [37], methodological limitations prevent definite conclusions [37, 38]. On the contrary, damage in the right hemisphere has been linked to a lack of awareness of mental abilities (anosognosia) [39], which may lead the patients to underestimate their true abilities. However, a study of high-grade glioma patients found that many patients are aware of their cognitive deficits after treatment with no difference in tumor laterality [12].
Postoperative improvement in glioblastoma patients may be a result of reduced mass effect, reduced peritumoral edema, termination of high-dose corticosteroids, psychological factors and more. However, the VLSM analysis of postoperative patient-reported cognitive change yielded no significant voxels, indicating that cognitive change may not be as location specific as the preoperative cognitive state. Still, from the descriptive maps, there seem to be several locations linked to postoperative improvement, except for the left central structures, where postoperative worsening was more often observed. A higher likelihood of surgery-induced damages in these areas may explain our finding. An alternative explanation might be that neurosurgeons have a more conservative surgical approach to centrally located tumors due to their potential critical impact on outcomes. However, our descriptive findings must be interpreted cautiously, and additional studies are needed to better understand these findings.
Cognitive function has been widely studied in neuropsychological tests [2, 4, 40], but the test results often differ from the patients’ experience [11, 41]. In this study, cognitive function was measured with the patient-reported questionnaire EORTC cognitive function subscale to ensure relevance for the patients. The outcome measure does not replace a neuropsychological test, but the subscale represents an approach to obtain outcome data about the patient’s self-perceived cognitive function. Patient-reported cognitive function was operationalized by the two questions about concentration and memory. Thus, we were not able to measure locations vulnerable to specific mental abilities, and a more comprehensive questionnaire may have detected more subtle impairments and yielded more detailed results. Also, despite the fairly large sample size, there were too few tumors in some voxels to be included in the analyses, and thus not all structures important for patient-reported cognitive function might have been detected. Still, there seems to be an anatomical relation between tumor location and self-reported cognitive function in glioblastoma patients, supporting a potential clinical validity of patient-reported measures of cognition.
The fairly large prospectively collected population-based sample is a major strength in this study, increasing the generalizability of our findings. Still, selection bias may have occurred, given some patients' lack of informed consent or nonresponding at 1 month follow-up. This is an unavoidable issue in glioma studies [42]. Furthermore, other factors important for patient-reported cognitive function may have affected our results, such as the use of corticosteroids and antiepileptics, oncological treatment, and tumor progression. Also, we did not register handedness, but the left hemisphere is dominant in 95% of right-handers and 70–80% of left-handers [6]. Another limitation is that several people contributed with tumor segmentations, which might have influenced the results. However, everyone who contributed has been trained in image interpretation by a neuroradiologist or an experienced glioma surgeon and image segmentations were reviewed by a neuroradiologist or the same neurosurgeon.
Although the VLSM method is increasingly used to measure the role of tumor location to functions, it has some disadvantages. First, registration of MRI images to the standardized MNI space can cause inaccuracies, especially in cases of significant mass effect and/or edema. Second, the tumors may functionally affect regions outside their radiological borders due to mass effects, edema, and their infiltrating and rapid tumor growth. Third, the heterogeneous distribution of tumors within the brain means that statistical power in many voxel-based analyses may be low. Since VLSM results can be vulnerable to false negatives in regions with few tumors, we decided to include descriptive maps. However, descriptive maps should be interpreted with caution. Still, as also argued by others, VLSM is the best available method to assess the relationship between location and functioning in brain tumor patients and allows valid conclusions [43].
Conclusion
We found the left hemisphere to be dominant for preoperative cognitive function from the patients’ perspective. More specifically, glioblastomas around the superior part of the left ventricle, the left lateral thalamus, the left caudate nucleus, and the left internal capsule by the arcuate fascicle were significantly associated with reduced patient-reported cognitive function before surgery. No areas were found to be significantly associated with patient-reported postoperative changes. Our findings suggest that there might be an anatomical relationship between patient-reported cognitive function and tumor location.
Data availability
The dataset generated during and/or analyzed during the current study are not publicly available due to privacy concerns but are available from the corresponding author on reasonable request.
References
Noll KR, Weinberg JS, Ziu M, Benveniste RJ, Suki D, Wefel JS (2015) Neurocognitive changes associated with surgical resection of left and right temporal lobe glioma. Neurosurgery 77:777–785. https://doi.org/10.1227/neu.0000000000000987
Dallabona M, Sarubbo S, Merler S, Corsini F, Pulcrano G, Rozzanigo U, Barbareschi M, Chioffi F (2017) Impact of mass effect, tumor location, age, and surgery on the cognitive outcome of patients with high-grade gliomas: a longitudinal study. Neurooncol Pract 4:229–240. https://doi.org/10.1093/nop/npw030
Noll KR, Ziu M, Weinberg JS, Wefel JS (2016) Neurocognitive functioning in patients with glioma of the left and right temporal lobes. J Neurooncol 128:323–331. https://doi.org/10.1007/s11060-016-2114-0
Habets EJJ, Kloet A, Walchenbach R, Vecht CJ, Klein M, Taphoorn MJB (2014) Tumour and surgery effects on cognitive functioning in high-grade glioma patients. Acta Neurochir (Wien) 156:1451–1459. https://doi.org/10.1007/s00701-014-2115-8
Sinha R, Stephenson JM, Price SJ (2020) A systematic review of cognitive function in patients with glioblastoma undergoing surgery. Neuro-Oncology Practice 7:131–142. https://doi.org/10.1093/nop/npz018
Lezak MD (2012) Neuropsychological assessment. Oxford University Press, Oxford
Kamada K, Todo T, Masutani Y, Aoki S, Ino K, R.T., Morita A, Saito N (2007) Visualization of the frontotemporal language fibers by tractography combined with functional magnetic resonance imaging and magnetoencephalography. J Neurosurg JNS 106: 90-98https://doi.org/10.3171/jns.2007.106.1.90
Luna LP, Sherbaf FG, Sair HI, Mukherjee D, Oliveira IB, Köhler CA (2021) Can Preoperative Mapping with Functional MRI Reduce Morbidity in Brain Tumor Resection? A Systematic Review and Meta-Analysis of 68 Observational Studies. Radiology 300:338–349. https://doi.org/10.1148/radiol.2021204723
Hamer PDW, Robles SG, Zwinderman AH, Duffau H, Berger MS (2012) Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol 30:2559–2565. https://doi.org/10.1200/jco.2011.38.4818
Cheng J-x, Liu B-l, Zhang X, Lin W, Zhang Y-q, Liu W-p, Zhang J-n, Lin H, Wang R, Yin H (2010) Health-related quality of life in glioma patients in China. BMC Cancer 10:1–8. https://doi.org/10.1186/1471-2407-10-305
Gehring K, Taphoorn MJB, Sitskoorn MM, Aaronson NK (2015) Predictors of subjective versus objective cognitive functioning in patients with stable grades II and III glioma. Neuro-Oncol Pract 2:20–31. https://doi.org/10.1093/nop/npu035
Giovagnoli AR, Meneses RF, Paterlini C, Silvani A, Boiardi A (2021) Cognitive awareness after treatment for high-grade glioma. Clin Neurol Neurosurg 210:106953. https://doi.org/10.1016/j.clineuro.2021.106953
Schei S, Solheim O, Salvesen Ø, Hjermstad MJ, Bouget D, Sagberg LM (2022) Pretreatment patient-reported cognitive function in patients with diffuse glioma. Acta Neurochir (Wien) 164:703–711. https://doi.org/10.1007/s00701-022-05126-9
Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF (2003) Voxel-based lesion–symptom mapping. Nat Neurosci 6:448–450. https://doi.org/10.1038/nn1050
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol 114:97–109. https://doi.org/10.1007/s00401-007-0243-4
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. JNCI: J Natl Cancer Inst 85:365–376. https://doi.org/10.1093/jnci/85.5.365
Fayers P, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A (2001) EORTC QLQ-C30 Scoring manual. European Organisation for Research and Treatment of Cancer. https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf. Accessed 21 Jul 2022
Mor V, Laliberte L, Morris JN, Wiemann M (1984) The Karnofsky performance status scale: an examination of its reliability and validity in a research setting. Cancer 53:2002–2007. https://doi.org/10.1002/1097-0142(19840501)53:9/3C2002::aid-cncr2820530933/3E3.0.co;2-w
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Ibañez FAL, Hem S, Ajler P, Vecchi E, Ciraolo C, Baccanelli M, Tramontano R, Knezevich F, Carrizo A (2011) A new classification of complications in neurosurgery. World Neurosurg 75:709–715. https://doi.org/10.1016/j.wneu.2010.11.010
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R (2012) 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30:1323–1341. https://doi.org/10.1016/j.mri.2012.05.001
Fyllingen EH, Stensjøen AL, Berntsen EM, Solheim O, Reinertsen I (2016) Glioblastoma Segmentation: Comparison of Three Different Software Packages. PLoS ONE 11:e0164891. https://doi.org/10.1371/journal.pone.0164891
Sagberg LM, Iversen DH, Fyllingen EH, Jakola AS, Reinertsen I, Solheim O (2019) Brain atlas for assessing the impact of tumor location on perioperative quality of life in patients with high-grade glioma: A prospective population-based cohort study. Neuroimage Clin 21:101658. https://doi.org/10.1016/j.nicl.2019.101658
Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL (2011) Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 54:313–327. https://doi.org/10.1016/j.neuroimage.2010.07.033
Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014) Permutation inference for the general linear model. Neuroimage 92:381–397. https://doi.org/10.1016/j.neuroimage.2014.01.060
Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, Ringelstein E-B, Henningsen H (2000) Handedness and hemispheric language dominance in healthy humans. Brain 123:2512–2518. https://doi.org/10.1093/brain/123.12.2512
Catani M, Jones DK, Ffytche DH (2005) Perisylvian language networks of the human brain. Ann Neurol 57:8–16. https://doi.org/10.1002/ana.20319
Fama R, Sullivan EV (2015) Thalamic structures and associated cognitive functions: Relations with age and aging. Neurosci Biobehav Rev 54:29–37. https://doi.org/10.1016/j.neubiorev.2015.03.008
Habets EJJ, Hendriks EJ, Taphoorn MJB, Douw L, Zwinderman AH, Vandertop WP, Barkhof F, De Witt Hamer PC, Klein M (2019) Association between tumor location and neurocognitive functioning using tumor localization maps. J Neurooncol 144:573–582. https://doi.org/10.1007/s11060-019-03259-z
Banerjee P, Leu K, Harris RJ, Cloughesy TF, Lai A, Nghiemphu PL, Pope WB, Bookheimer SY, Ellingson BM (2015) Association between lesion location and language function in adult glioma using voxel-based lesion-symptom mapping. NeuroImage: Clinical 9:617–624. https://doi.org/10.1016/j.nicl.2015.10.010
Zhao L, Biesbroek JM, Shi L, Liu W, Kuijf HJ, Chu WW, Abrigo JM, Lee RK, Leung TW, Lau AY (2018) Strategic infarct location for post-stroke cognitive impairment: A multivariate lesion-symptom mapping study. J Cereb Blood Flow Metab 38:1299–1311. https://doi.org/10.1177/0271678x17728162
Weaver NA, Kuijf HJ, Aben HP, Abrigo J, Bae H-J, Barbay M, Best JG, Bordet R, Chappell FM, Chen CPLH, Dondaine T, van der Giessen RS, Godefroy O, Gyanwali B, Hamilton OKL, Hilal S, HuengesWajer IMC, Kang Y, Kappelle LJ, Kim BJ, Köhler S, de Kort PLM, Koudstaal PJ, Kuchcinski G, Lam BYK, Lee B-C, Lee K-J, Lim J-S, Lopes R, Makin SDJ, Mendyk A-M, Mok VCT, Oh MS, van Oostenbrugge RJ, Roussel M, Shi L, Staals J, del C Valdés-Hernández M, Venketasubramanian N, Verhey FRJ, Wardlaw JM, Werring DJ, Xin X, Yu K-H, van Zandvoort MJE, Zhao L, Biesbroek JM, Biessels GJ (2021) Strategic infarct locations for post-stroke cognitive impairment: a pooled analysis of individual patient data from 12 acute ischaemic stroke cohorts. Lancet Neurology 20:448–459. https://doi.org/10.1016/S1474-4422(21)00060-0
Graff-Radford J, Williams L, Jones DT, Benarroch EE (2017) Caudate nucleus as a component of networks controlling behavior. 2192–2197. https://doi.org/10.1212/WNL.0000000000004680
Gainotti G (1972) Emotional Behavior and Hemispheric Side of the Lesion. Cortex 8:41–55. https://doi.org/10.1016/S0010-9452(72)80026-1
Robinson RG, Kubos KL, Starr LB, Rao K, Price TR (1984) Mood disorders in stroke patients. Importance of location of lesion. Brain 107:81–93. https://doi.org/10.1093/brain/107.1.81
Rooney AG, Carson A, Grant R (2010) Depression in Cerebral Glioma Patients: A Systematic Review of Observational Studies. JNCI: J Natl Cancer Inst 103:61–76. https://doi.org/10.1093/jnci/djq458
Rooney AG, Brown PD, Reijneveld JC, Grant R (2014) Depression in glioma: a primer for clinicians and researchers. J Neurol Neurosurg Psychiatry 85:230–235. https://doi.org/10.1136/jnnp-2013-306497
Orfei MD, Robinson RG, Prigatano GP, Starkstein S, Rüsch N, Bria P, Caltagirone C, Spalletta G (2007) Anosognosia for hemiplegia after stroke is a multifaceted phenomenon: a systematic review of the literature. Brain 130:3075–3090. https://doi.org/10.1093/brain/awm106
Talacchi A, Santini B, Savazzi S, Gerosa M (2011) Cognitive effects of tumour and surgical treatment in glioma patients. J Neurooncol 103:541–549. https://doi.org/10.1007/s11060-010-0417-0
Pranckeviciene A, Deltuva VP, Tamasauskas A, Bunevicius A (2017) Association between psychological distress, subjective cognitive complaints and objective neuropsychological functioning in brain tumor patients. Clin Neurol Neurosurg 163:18–23. https://doi.org/10.1016/j.clineuro.2017.10.007
Walker M, Brown J, Brown K, Gregor A, Whittle I, Grant R (2003) Practical problems with the collection and interpretation of serial quality of life assessments in patients with malignant glioma. J Neurooncol 63:179–186. https://doi.org/10.1023/A:1023900802254
Duffau H (2011) Do brain tumours allow valid conclusions on the localisation of human brain functions? Cortex 47:1016–1017. https://doi.org/10.1016/j.cortex.2010.11.010
Acknowledgements
We would like to thank Camilla Brattbakk, Linda Nordtvedt, and Even Hovig Fyllingen for assisting in data collection.
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital) The article was funded by the Faculty of Medicine and Health Sciences at the Norwegian University of Science and Technology and the Norwegian national advisory unit for Ultrasound and image-guided therapy (USIGT).
Author information
Authors and Affiliations
Contributions
Conception and design: all authors; Data collection; Lisa Millgård Sagberg, Ole Solheim, Stine Schei; Statistical analysis: Lars Eirik Bø, Stine Schei; Interpretation of data: Stine Schei, Ole Solheim, Lisa Millgård Sagberg; Drafting the article: Stine Schei, Ole Solheim, Lisa Millgård Sagberg, Lars Eirik Bø; Critically revising the article: all authors; Revised submitted version of manuscript: all authors; Approved the final version of the manuscript on behalf of all authors: Stine Schei; Study supervision: Ole Solheim, Lisa Millgård Sagberg.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Regional Committee for Medical Research Ethics (reference number 2013/1348) and was in accordance with the Helsinki Declaration. All patients provided written informed consent.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Video 1 Axial maps of preoperative tumor distribution, descriptive maps with mean preoperative patient-reported cognitive function, and preoperative VLSM maps. N = 162 (MP4 23917 KB)
Supplementary Video 2 Axial maps of preoperative tumor distribution for the sample with postoperative follow-up and descriptive maps with mean postoperative change in patient-reported cognitive function at 1 month. N = 99 (MP4 12868 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schei, S., Sagberg, L.M., Bø, L.E. et al. Association between patient-reported cognitive function and location of glioblastoma. Neurosurg Rev 46, 282 (2023). https://doi.org/10.1007/s10143-023-02177-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02177-z