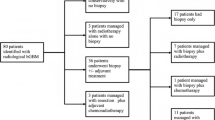
Abstract
Butterfly glioblastoma (bGBM) is a grade 4 glioma with a poor prognosis. Surgical treatment of these cancers has been reviewed in the literature with some recent studies supporting resection as a safe and effective treatment instead of biopsy and adjuvant therapy. This meta-analysis was designed to determine whether there are significant differences in overall survival (OS) and postoperative neurologic deficits (motor, speech, and cranial nerve) following intervention in patients who underwent tumor resection as part of their treatment, compared to patients who underwent biopsy without surgical resection. A literature search was conducted using PubMed (National Library of Medicine) and Embase (Elsevier) to identify articles from each database’s earliest records to May 25, 2021, that directly compared the outcomes of biopsy and resection in bGBM patients and met predetermined inclusion criteria. A meta-analysis was conducted to compare the effects of the two management strategies on OS and postoperative neurologic deficits. Six articles met our study inclusion criteria. OS was found to be significantly longer for the resection group at 6 months (odds ratio [OR] 2.94, 95% confidence interval [CI] 1.23–7.05) and 12 months (OR 3.75, 95% CI 1.10–12.76) than for the biopsy group. No statistically significant differences were found in OS at 18 and 24 months. Resection was associated with an increased rate of postoperative neurologic deficit (OR 2.05, 95% CI 1.02–4.09). Resection offers greater OS up to 1 year postintervention than biopsy alone; however, this comes at the cost of higher rates of postoperative neurologic deficits.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Ostrom QT, Cioffi G, Gittleman H et al (2019) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol 21(Suppl 5):v1–v100
Steltzer KJ, Sauvé KI, Spence AM, Griffin TW, Berger MS (1997) Corpus callosum involvement as a prognostic factor for patients with high-grade astrocytoma. Int J Radiat Oncol Biol Phys 38(1):27–30
Chaichana KL, Jusue-Torres I, Lemos AM et al (2014) The butterfly effect on glioblastoma: is volumetric extent of resection more effective than biopsy for these tumors? J Neurooncol 120(3):625–634
Burks JD, Bonney PA, Conner AK et al (2017) A method for safely resecting anterior butterfly gliomas: the surgical anatomy of the default mode network and the relevance of its preservation. J Neurosurg 126(6):1795–1811
Chojak R, Koźba-Gosztyła M, Słychan K et al (2021) Impact of surgical resection of butterfly glioblastoma on survival: a meta-analysis based on comparative studies. Sci Rep 11(1):13934
Dayani F, Young JS, Bonte A et al (2018) Safety and outcomes of resection of butterfly glioblastoma. Neurosurg Focus 44(6):E4
Franco P, Delev D, Cipriani D et al (2021) Surgery for IDH1/2 wild-type glioma invading the corpus callosum. Acta Neurochir (Wien) 163(4):937–945
Kim YJ, Lee DJ, Park CK, Kim IA (2019) Optimal extent of resection for glioblastoma according to site, extension, and size: a population-based study in the temozolomide era. Neurosurg Rev 42(4):937–950
Niare M, Desrousseaux J, Cavandoli C, Virak V, Sacko O, Charni S, Roux FE (2022) Outcome of glioblastoma resection in patients 80 years of age and older. Acta Neurochir (Wien) 164(2):373–383
Opoku-Darko M, Amuah JE, Kelly JJP (2018) Surgical resection of anterior and posterior butterfly glioblastoma. World Neurosurg 110:e612–e620
Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115(1):3–8
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Balaña C, Capellades J, Teixidor P et al (2007) Clinical course of high-grade glioma patients with a “biopsy-only” surgical approach: a need for individualised treatment. Clin Transl Oncol 9(12):797–803
Dziurzynski K, Blas-Boria D, Suki D et al (2012) Butterfly glioblastomas: a retrospective review and qualitative assessment of outcomes. J Neurooncol 109(3):555–563
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 339(21):b2535–b2535
Sterne JA, Hernán MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Cochran WG (1950) The comparison of percentages in matched samples. Biometrika 37(3–4):256–266
DerSimonian R, Laird N (2015) Meta-analysis in clinical trials revisited. Contemp Clin Trials 45(Pt A):139–145
Abdullah KG, Ramayya A, Thawani JP et al (2015) Factors associated with increased survival after surgical resection of glioblastoma in octogenarians. PLoS ONE 10(5):e0127202
Chaichana KL, Chaichana KK, Olivi A et al (2011) Surgical outcomes for older patients with glioblastoma multiforme: preoperative factors associated with decreased survival Clinical article. J Neurosurg 114(3):587–594
Gupta M, Bansal S, Pruthi DS, Saini M, Shirazi N, Ahmad M (2018) Prognostic factors in elderly patients with high-grade gliomas: a retrospective analysis of 24 cases. J Neurosci Rural Pract 9(3):312–316
Awad AW, Karsy M, Sanai N et al (2017) Impact of removed tumor volume and location on patient outcome in glioblastoma. J Neurooncol 135(1):161–171
Chen KT, Wu TW, Chuang CC et al (2015) Corpus callosum involvement and postoperative outcomes of patients with gliomas. J Neurooncol 124(2):207–214
Shinoda J, Sakai N, Murase S, Yano H, Matsuhisa T, Funakoshi T (2001) Selection of eligible patients with supratentorial glioblastoma multiforme for gross total resection. J Neurooncol 52(2):161–171
Acknowledgements
We thank Paul H. Dressel BFA for formatting the figures and Carrie Owens MSILS and Debra J. Zimmer for editorial support.
Author information
Authors and Affiliations
Contributions
Conception and design: Soliman, A Khan, Fenstermaker, Plunkett.
Acquisition of data: Azmy, Gilbert, Goliber, Szczecinski, Durrani, Burke.
Interpretation of data: all authors.
Drafting the manuscript: Azmy, Soliman.
Critically revising the manuscript: all authors.
Reviewed submitted version of manuscript: all authors.
Statistical analysis: S Khan, Salem.
Study supervision: Siddiqui, Fenstermaker, Plunkett.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The University at Buffalo Institutional Review Board determined that this study was not research involving human subjects and that their review and approval was not required. Additionally, consent to participate was not required.
Human and animal ethics
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Fenstermaker is a co-founder and equity holder in MimiVax, LLC, which develops immunotherapeutic agents for treatment of glioblastoma.
Dr. A. Khan has received research grants from the Scoliosis Research Society to study scoliosis in Chiari patients and from the Neurosurgical Research and Education Foundation (NREF) to study bupivacaine pain pumps in patients undergoing spinal deformity correction.
Dr. Davies consulting fees, payment, or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events; support for attending meetings and/or travel: Medtronic. Patents planned, issued, or pending: QAS.ai. Participation on a Data Safety Monitoring Board or Advisory Board: NIH NIHDS Strokenet. Stock or stock options: Synchron, Cerebrotech, QAS.ai.
Dr. Levy shareholder/ownership interest: NeXtGen Biologics, RAPID Medical, Claret Medical, Cognition Medical, Imperative Care, Rebound Therapeutics, StimMed, Three Rivers Medical; Patent: Bone Scalpel; Honorarium for Training and Lectures: Medtronic, Penumbra, MicroVention, Integra, Consultant: Clarion, GLG Consulting, Guidepoint Global, Imperative Care, Medtronic, StimMed, Misionix, Mosiac; Chief Medical Officer: Haniva Technology; National PI: Medtronic- Steering Committees for SWIFT Prime and SWIFT Direct Trials; Site PI Study: MicroVention (CONFIDENCE Study) Medtronic (STRATIS Study-Sub 1); Advisory Board: Stryker (AIS Clinical Advisory Board), NeXtGen Biologics, MEDX, Cognition Medical; Endostream Medical, IRRAS AB (Consultant/Advisory Board, Medical Legal Review): render medical/legal opinions as an expert witness; leadership or fiduciary roles in other board society, committee, or advocacy group, paid and unpaid: CNS, ABNS, UBNS.
Dr. Mullin is involved with clinical research for Cerapedics. He receives research funding from AOSpine North America (AOSNA) and the Research Committee Award #87639; and from Medtronic External Research Program Health Professionals, ERP ID#2020–12271.
Dr. Plunkett is a non-paid consultant for Medtronic and a contributing investigator for the Medtronic Product Registry.
Dr. Pollina is involved with surgical training for Medtronic and serves as a consultant for and receives royalties from ATEC Spine.
Dr. Siddiqui consulting fees: Amnis Therapeutics, Apellis Pharmaceuticals, Inc., Boston Scientific, Canon Medical Systems USA, Inc., Cardinal Health 200, LLC, Cerebrotech Medical Systems, Inc., Cerenovus, Cerevatech Medical, Inc., Cordis, Corindus, Inc., Endostream Medical, Ltd, Imperative Care, InspireMD, Ltd., Integra, IRRAS AB, Medtronic, MicroVention, Minnetronix Neuro, Inc., Peijia Medical, Penumbra, Q’Apel Medical, Inc., Rapid Medical, Serenity Medical, Inc., Silk Road Medical, StimMed, LLC, Stryker Neurovascular, Three Rivers Medical, Inc., VasSol, Viz.ai, Inc. Leadership or fiduciary role in other board, society, committee, or advocacy group: Secretary – Board of the Society of NeuroInterventional Surgery 2020–2021, Chair –Cerebrovascular Section of the AANS/CNS 2020–2021. Stock or stock options: Adona Medical, Inc., Amnis Therapeutics, Bend, IT Technologies, Ltd., BlinkTBI, Inc, Cerebrotech Medical Systems, Inc., Cerevatech Medical, Inc., Cognition Medical, CVAID Ltd., E8, Inc., Endostream Medical, Ltd, Galaxy Therapeutics, Inc., Imperative; Care, Inc., InspireMD, Ltd., Instylla, Inc., International Medical Distribution Partners, Launch NY, Inc.; Neurolutions, Inc., NeuroRadial Technologies, Inc., NeuroTechnology Investors, Neurovascular Diagnostics, Inc., Peijia; Medical, PerFlow Medical, Ltd., Q’Apel Medical, Inc., QAS.ai, Inc., Radical Catheter Technologies, Inc., Rebound Therapeutics Corp. (Purchased 2019 by Integra Lifesciences, Corp), Rist Neurovascular, Inc. (Purchased 2020 by Medtronic), Sense Diagnostics, Inc., Serenity Medical, Inc., Silk Road Medical, Sim & Cure, SongBird Therapy, Spinnaker Medical, Inc., StimMed, LLC, Synchron, Inc., Three Rivers Medical, Inc., Truvic Medical, Inc., Tulavi Therapeutics, Inc., Vastrax, LLC, VICIS, Inc., Viseon, Inc. Other financial or non-financial interests: National PI/Steering Committees: Cerenovus EXCELLENT and ARISE II Trial; Medtronic SWIFT PRIME, VANTAGE, EMBOLISE, and SWIFT DIRECT Trials; MicroVention FRED Trial & CONFIDENCE Study; MUSC POSITIVE Trial; Penumbra 3D Separator Trial, COMPASS Trial, INVEST Trial, MIVI neuroscience EVAQ Trial; Rapid Medical SUCCESS Trial; InspireMD C-GUARDIANS IDE Pivotal Trial.
Dr Snyder consulting fees: Boston Scientific, Canon Medical Systems USA, Inc., MicroVention, Medtronic, Stryker Neurovascular. Payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing, or educational event: Canon Medical Systems USA Inc. Stock or stock options: Boston Scientific, Access Closure Inc., Niagara Gorge Medical.
All other authors have no personal, financial, or institutional interest in the materials or devices described in this manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Previous Presentation
No
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soliman, M.A., Khan, A., Azmy, S. et al. Meta-analysis of overall survival and postoperative neurologic deficits after resection or biopsy of butterfly glioblastoma. Neurosurg Rev 45, 3511–3521 (2022). https://doi.org/10.1007/s10143-022-01864-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-022-01864-7