Abstract
Indications for surgery of pineal cysts without ventriculomegaly are still under debate. In view of the limited data for pineal cyst resection in the absence of hydrocephalus, and the potential risk of this approach, we have analyzed our patient cohort focusing on strategies to avoid complications according to our experience in a series of 73 pineal cyst patients. From 2003 to 2015, we reviewed our database retrospectively for all patients operated on a pineal cyst. Furthermore, we prospectively collected patients from 2016 to 2020. In summary, 73 patients with a pineal cyst were treated surgically between 2003 and 2020. All patients were operated on via a microscopic supracerebellar-infratentorial (SCIT) approach. The mean follow-up period was 26.6 months (range: 6–139 months). Seventy-three patients underwent surgery for a pineal cyst. An absence of enlarged ventricles was documented in 62 patients (51 female, 11 male, mean age 28.1 (range 4–59) years). Main presenting symptoms included headache, visual disturbances, dizziness/vertigo, nausea/emesis, and sleep disturbances. Complete cyst resection was achieved in 59/62 patients. Fifty-five of 62 (89%) patients improved after surgery with good or even excellent results according to the Chicago Chiari Outcome Scale, with complete or partial resolution of the leading symptoms. Pineal cysts resection might be an indication in certain patients for surgery even in the absence of ventriculomegaly. The high percentage of postoperative resolution of quality-of-life impairing symptoms in our series seems to justify surgery. Preoperatively, other causes of the leading symptoms have to be excluded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance imaging (MRI) reveals pineal cysts in 1.8 to 4.3% of healthy subjects [1,2,3,4]. Autopsy studies suggest that up to 40% of the general population harbors a pineal cyst [5,6,7]. Although usually asymptomatic, pineal cysts can grow leading to mass effect on the surrounding structures (tectum, aqueduct, venous structures) and thereby necessitate treatment [2, 8,9,10,11,12,13,14,15,16,17,18,19].
Due to the high prevalence of pineal cysts with nonspecific symptoms, the discussion about the relation of the symptoms to the pineal cysts is ongoing [6, 20,21,22,23]. Nowadays, the management of patients who present only with headache but without neurological symptoms and a pineal cyst in the absence of hydrocephalus is not clearly defined. Some authors suggest that in the absence of obstructive hydrocephalus or tectal compression, symptoms should not be attributed to the pineal cyst and these patients should be managed conservatively [20]. Otherwise, it is postulated that pineal cyst may cause intermittent obstruction of the Sylvian aqueduct or compression of critical neural structures, making resection reasonable after thorough preoperative examination [2, 6, 12, 24,25,26,27,28,29]. Pineal abnormalities may play a critical role in sleep disturbances and even in major depressive disorders [30]. Due to ethical reasons, a randomized controlled trial will be not acceptable to clarify more insights in the role of surgical therapy for pineal cysts associated with “unspecific symptoms.” Large prospective studies are limited, whereas prospective data collection of case series seems to be a proper tool [31].
Therefore, we present our experience with the resection of pineal cysts in the absence of ventriculomegaly to roll out the indication for surgery and to report on complications and their avoidance.
Patients and methods
The study protocol has been approved by the local ethics committee. It includes a retrospective review of prospectively collected data and records of the patients who underwent a surgery for a pineal cyst without ventriculomegaly from 2003 to 2015 as well as a follow-up clinical examination. All subjects gave written informed consent. Furthermore, we collected data prospectively from 2016 to 2020. We studied our consecutive case series from a single academic institution regarding demographic data, presenting symptoms, radiological characteristics, surgical approach, extent of resection, perioperative complications, as well as clinical and radiological follow-up. Other possible and obvious causes of the leading symptoms had been ruled out by consultations (e.g., ophthalmology, neurology, neuropediatrics, internal medicine, psychiatry, and/or psychology). The investigations were carried out in the referring center. Otherwise, we completed the missing investigations in our institution within a structured preoperative work-up. Intracranial pressure (ICP) monitoring was not performed prior to surgery.
In view of the still vague indication for surgery in pineal cysts without hydrocephalus, we discussed extensively the decision with our patients individually. Typical symptoms considered during decision-making were headache associated with symptoms denoting elevated intracranial pressure, i.e., nausea and/or emesis, visual disturbance (blurring of vision, upward gaze palsy) resulting from possible tectal compression. Other, more unrelated symptoms were sometimes described by patients (see Table 1 for details).
Importantly, the difficulty in attributing (all) their symptoms to the pineal cyst, and that their symptoms or at least part of them may have resulted from a temporary elevation in intracranial pressure was extensively discussed with the patients. Especially, that they may not benefit from surgery, and on the contrary, being exposed to the risks of surgery.
Surgery
Preoperatively, transesophageal echocardiography was performed to rule out an open foramen ovale and to minimize the risks from air embolism. The surgical notes were reviewed regarding the approach, characteristics of cyst removal, and perioperative complications as mentioned above.
Postoperative evaluation
All patients from 2016 to 2020 were assessed prospectively after surgery and before discharge, 3 and 6 months after surgery and then yearly. All patients were followed finally until April 2021. The examinations were carried out by a neurosurgeon who was not directly involved in the surgery. The Chicago Chiari Outcome Scale [32] (CCOS, see Table 2) was calculated.
Májovsky et al. used this score to assess the clinical outcome of patients with symptomatic pineal cysts as they have some similarities with patients with Chiari malformation [33].
Radiological evaluation
The diameter of the pineal cyst was measured as maximum width of the cyst in a mid-sagittal T2-weighed or constructive interference in steady state (CISS) MR image.
Surgical details
Approach and positioning
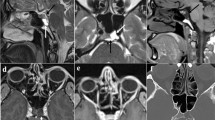
A microsurgical total cyst resection was performed via a supracerebellar-infratentorial approach (SCIT). Initially, we used the standard midline approach (n = 15). However, since 2015 we have used the less invasive small unilateral paramedian approach. This approach has the advantage that the viewing trajectory to the pineal region is not as steep as in the midline approach (see Fig. 1G).
In 53 individuals, a semi-sitting positioning has been performed. Regarding a persistent foramen ovale (in preoperative transesophageal echocardiography), surgery was done in prone position (n = 9) (Fig. 1C).
Midsagittal MR CISS before (A) and after surgery (B) demonstrating a pineal cyst leading to a narrowed Sylvian aqueduct (arrow) without hydrocephalus. Patient complaining of longstanding and increasing headache attacks. Post-operative MR CISS (B) midsagittal image: complete cyst resection; open Sylvian aqueduct. Craniotomy reaching the transverse sinus (arrow-head). Symptoms resolved completely. C Semi-sitting position. Contrast-enhanced T1-weighted MR (coronal (D, E), axial (F)): left-sided predominant sinus and bridging vein; decision for approach on right side with the higher-rising sinus. Variations of steepness of tent: paramedian (G) viewing trajectory to the pineal region is not as steep as in the midline (H) approach
Specific anesthesiological considerations
Specific anesthesiological preparations include central venous line and permanent transesophageal echocardiographic monitoring to offer the direct control of an ongoing air embolism.
Preoperative planning and radiographic landmarks
We use anatomical landmarks to perform the approach. Navigation was not applied.
We look carefully for any side predominance and height of the transverse sinus as well as the location of bridging veins. In children, we look additionally for an open occipital sinus. If one transverse sinus is high rising at the coronal MR image, we approach from that side because the angle of view is not so steep.
MR imaging with contrast-enhanced T1-weighted and T2-weighted images in axial and coronal plane shows the sinus and veins very well (see Fig. 1D–F). The steepness of the tent is very variable (see Fig. 1G, H).
We prefer a paramedian 2-cm wide supracerebellar approach on the non-dominant side of a high-rising transverse sinus, ideally with smaller or absent bridging veins.
Skin incision, bone flap
A straight 5–6 cm long skin incision beginning 1.5 cm over the level of the external occipital protuberance 2 cm paramedian to the midline is made. The fascia is incised and with careful sharp and blunt dissection we look for the major occipital nerve (Fig. 2A). Then, a 2–3 cm paramedian bone flap exposing the lower third of the transverse sinus is raised (Fig. 2B). After the craniotomy, a jugular compression test is performed to make sure that no venous leak exists.
A Skin incision; greater occipital nerve (N). B Craniotomy exposing the transverse sinus. C Dural incision alongside the transverse sinus. D and E Dissection of bridging vein (V). F Pineal cyst (C) surrounded by thick arachnoid (A). G Bimanual dissection. H Resected pineal cyst (C). I + J Endoscopic view into the third ventricle showing massa intermedia (M) and posterior commissure (PC). K Endoscopic view to the roof of the third ventricle with a 45° endoscope shows the large internal cerebral veins (IV). L Microscopic view of the resection cavity showing gross total cyst resection. M Preservation of the bridging vein after cyst removal. N Dural closure. O Bone flap fixation with miniplates
Opening of the dura, CSF drainage, and bridging veins
The dura is incised below and parallel to the transverse sinus (Fig. 2C). Dural tacking stitches are placed to elevate the upper dural edge. This allows a view along the tent without any active retraction because of cerebellar retraction by gravity. Spatulas are not required. Careful covering of bone and dural edges after meticulous hemostasis is mandatory. If somehow possible, we avoid occluding even smaller veins. If a larger vein is within the approach trajectory to the pineal gland, we dissect the vein from the cerebellar surface to allow a little bit of cerebellar retraction by gravity to enable access to the pineal cyst while avoiding vein sacrifice (Fig. 2D, E, F).
The arachnoid covering the pineal region is always thick. Before arachnoid incision, the large veins such as vein of Galen and Rosenthal need to be identified. If they are collapsed, jugular compression should be performed. After opening of the quadrigeminal cistern, CSF is drained. Surgeon’s patience while draining enough CSF is a key step for a gentle operation.
Dissection of the cyst
The internal cerebral and basal veins and many of their tributaries converge at the great cerebral vein (of Galen) in the posterior incisural space. The vein of the cerebellomesencephalic fissure crosses the quadrigeminal cistern (ambient cistern) and drains directly or via superior vermian vein to the vein of Galen. Arterial tributaries are defined as P3 segment of PCA (posterior cerebral artery) and also enter the quadrigeminal cistern [34]. After opening of the quadrigeminal cistern (ambient cistern), the cyst is exposed by meticulous sharp dissection (Fig. 2G). Always, a good arachnoid dissection plane can be found around the cyst. The cyst is incised and collapsed. Typically, the cysts contain a yellowish fluid. The cyst wall is grasped with a small tumor forceps and the arachnoid plane is dissected with an anatomical forceps (traction-counter traction technique) [35]. Small feeding and draining vessels are coagulated and cut. The cyst is dissected from superior and inferior colliculi (quadrigeminal plate) beneath, from the thalamus laterally, and from habenula and posterior commissure anteriorly. After cyst removal (Fig. 2H), a jugular compression test is done to detect potential venous bleeding sources.
Endoscopic visualization (Fig. 2I–K) incl. 30° and 45° optics is of help to look to surrounding structures. However, in view of the usually narrow surgical corridor and the intended preservation of bridging veins, we do not use a purely endoscopic technique since the risk of damaging the veins is much higher because they are not in the visual field of the endoscope.
Closure
The dura is closed with a running suture (Fig. 2N). Usually, we add sealants (e.g., Hemopatch®, Baxter; Tachosil®, Takeda) to prevent any cerebrospinal fluid (CSF) leak via the small stitch channels. Rarely, we use a small muscle patch in larger dural defects. Interestingly, despite the opening of the third ventricle, we rarely observed (see Table 3) CSF leaks after surgery. The bone flap is fixed with titanium miniplates (Fig. 2O). The incision is closed in layers.
Results
Patients and presenting symptoms
Around 500 patients with pineal cysts were referred to our institution for evaluation from 2003 to 2020. Seventy-three patients underwent surgery for a pineal cyst. An absence of enlarged ventricles was documented in 62 patients (51 female, 11 male, mean age 28.1 years (± 12.15 years), range 4–59 years).
Presenting symptoms included headache (61/62), visual disturbances (23/62), dizziness/vertigo (24/62), nausea/emesis (41/62), and sleep disturbances (17/62). All the neuro-ophthalmologic examinations could rule out any objective preoperative disturbances.
Radiological findings
Preoperative mid-sagittal MRI (CISS or T2-weighted) usually demonstrated narrowing but no occlusion of the aqueduct due to the pineal cyst (Fig. 1A). The cyst size ranged from 7 to 27 mm (mean 14.87 mm) in maximal diameter. Enlarged ventricles were not observed. There was radiological evidence of cyst enlargement in preoperative follow-up MRI series in 6 of 62 patients. MRI obtained 1 day after surgery demonstrated no cyst remnant in all patients. Furthermore, there was no cyst recurrence during the follow-up period (mean 26.6 months (range: 6–139 months)).
Clinical outcome
Fifty-five of 62 (89%) patients improved after surgery with good or even excellent results according to the Chicago Chiari Outcome Scale (CCOS ≥ 12), with complete or partial resolution of the leading symptoms within a mean follow-up period of 26.6 months (range: 6–139 month). Details of outcome are shown in Table 1.
The average hospital stay was 7 days (± 3.5 days, range 5–22 days). The longer hospital stays were due to completion of the preoperative investigations. All patients were discharged to their homes. Neither direct referrals to rehabilitation centers nor long-term care facilities were needed.
Surgery
The semi-sitting position was used in 53 patients. Otherwise, we operated them in prone position in case of persistent oval foramen (n = 9).
In all patients, a microsurgical cyst resection was performed via a supracerebellar-infratentorial approach (SCIT). Total cyst resection could be achieved in 59 of 62 patients. Initially, we used the standard midline approach (n = 15). However, since 2015 we have used a less invasive small unilateral paramedian approach (n = 47).
The operation time was in average 211 (± 51) min (range 108 to 346 min).
Peri- and postoperative complications
Air embolism occurred in 8 patients. In only 2 of these patients, we encountered a significant air embolism with circulatory impairment during surgery which was managed with jugular vein compression to visualize the venous leak, and aspiration of air from the heart via the central line. In the other 6 patients, air embolism was only detected by the cardiac ultrasound. In all patients, air embolism had no sequelae. Intraoperative major surgical complications were not observed.
Pathology approved all resected cysts as simple pineal cysts without evidence of malignancy.
Eleven patients suffered from transient visual problems after surgery (diplopia in 5 patients, blurring of vision in 4 patients, nystagmus (n = 1), and 1 visual field defect). However, a detailed neuro-ophthalmological examination after surgery did not demonstrate any deficit in these patients. These symptoms resolved completely within 2–4 weeks after surgery in all patients.
One patient suffered from a wound infection and was managed conservatively.
Two subcutaneous CSF effusions occurred and resolved spontaneously. A postoperative hydrocephalus was not observed.
Postoperatively, analgetic medications have been used stepwise according to the WHO guidelines. In 2 patients, who had problems to fall into sleep, melatonin was administered for 2 to 4 weeks. No patient received long-term melatonin medication.
Further detailed information is shown in Table 3.
Discussion
To our knowledge, this series is the largest surgical series of patients who underwent resection of a simple pineal cyst without hydrocephalus. Most of our patients showed a good to excellent outcome which is in accordance with the literature.
Evaluating CCOS, in our series 55 of 62 (89%) patients reported good outcome with more than 11 points. In all patients, ventriculomegaly was not present suggesting that the absence of ventriculomegaly and/or tectum induced visual disturbances (Parinaud’s syndrome) is not a contraindications for surgery. Therefore, a surgical intervention should not be generally refused in case of an absent ventriculomegaly in patients with leading symptoms indicating a possible temporary increase of intracranial pressure.
Pineal cysts rarely cause symptoms [20, 36, 37]. They are usually assumed as incidental findings and were often identified during workup for headache. Mostly, they are not the cause of symptoms [20, 21, 38, 39]. According to the literature, large pineal cysts are considered to be potentially symptomatic lesions [6] while causing ventriculomegaly due to compression of the Sylvian aqueduct or Parinaud’s syndrome due to tectal compression [6, 10, 12, 15, 24, 25, 28, 29, 40,41,42].
Therefore, the discussion about the best management of patients with a pineal cyst without clear signs and symptoms of intracranial hypertension or symptomatic mass effect is still ongoing. There are some case series describing surgical results in pineal cysts without hydrocephalus or tectal compression. Kalani et al. [6] reported in 17 of 18 patients with resection of pineal cysts a resolution or improvement of their presenting symptoms. The authors suggest that the absence of ventriculomegaly and Parinaud’s syndrome are not absolute contraindications to surgical intervention.
Our previous results (series of 43 patients operated on pineal cyst without enlarged ventricles) could already demonstrate good to excellent clinical results [43]. In this period (2003 to 2018), we examined and followed 440 patients with pineal region pathologies according to Diagnosis Related Groups (DRG) codes.
However, we should clearly state that most pineal cysts are asymptomatic and do not require treatment. It is illustrated by Al-Holou and coworkers [20] who reviewed in one of the largest studies more than 48,000 adult patients undergoing brain MRI: they discovered 478 incidental pineal cysts greater than 5 mm on MRI. Headache was presented in 21% of them, but no cysts were considered as symptomatic, and surgical intervention was not indicated. During follow-up, 99% of cysts remained stable or even decreased in size.
Obviously, patients should be thoroughly investigated before deciding for a surgical resection in case of such a benign lesion and in view of the potential risk in this approach. Nevertheless, we believe that our results indicate that surgery may be an option after careful selection of patients to offer the possibility of marked relief of their symptoms and better quality of life in a small subset of pineal cyst patients.
Similar results were presented by Eide and Ringstad [44] who collected 27 patients managed surgically for non-hydrocephalic pineal cysts over a 10-year period.
The compression of the internal cerebral veins, vein of Rosenthal, or vein of Galen may influence the severity of symptoms in subjects with pineal cysts [45]. The resulting central venous hypertension might cause mild edema within the territories drained by the affected veins. MRI biomarkers indicating central venous hypertension have recently been reported [46].
Májovsky et al. reported over 110 patients with simple pineal cysts. The most common presenting symptoms were tension headache (62.7%), vertigo (16.4%), migraine (12.7%), syncope (10.9%), nausea (8.2%), and diplopia (8.2%). Twenty-one patients underwent pineal cyst resection; 20 patients (95.2%) reported some improvement in their presenting symptoms, and 10 patients (47.6%) were completely free after the surgery [33]. Their results of the surgical group are comparable to our results after surgery. We reported in 95% a good outcome after surgery and a very low incidence of complications [43]. Constantly, we can also demonstrate a low rate of complications with our recent cohort.
Recently, Masina et al. published a meta-analysis of the published literature addressing the surgical treatment of symptomatic pineal cysts without hydrocephalus. They concluded that although the results support the role of surgery in the management of this entity, they have to be interpreted with a great deal of caution as the current evidence is limited, consisting only of case reports and retrospective surgical series. Inherent to such studies are inhomogeneity and incompleteness of data, selection bias, and bias related to assessment of outcome carried out by the treating surgeon in the majority of cases. Prospective studies with patient-reported and objective outcome assessment are needed to provide higher level of evidence [47].
Some aspects of the surgical approach have to be discussed.
The SCIT represents an extraparenchymatous approach via a natural corridor. Alternatives might be the occipital transtentorial approach (OTT) and the occipital bi-transtentorial/falcine approach [34] or—only in case of enlarged ventricles—the frontal endoscopic approach. We would not suggest the OTT because the pineal cyst is located under the tent and veins. Why should we come from above with the need to cut the tent? Furthermore, there is a risk of postoperative hemianopia [48,49,50]. Also, pure endoscopic SCIT has been described [2]. However, the narrow surgical corridor in our minimally invasive paramedian approach does not allow an unrestricted manual dissection with an endoscope.
We prefer the (semi-) sitting position (Fig. 2): advantages are proper gravity-induced cerebellar retraction and fluid drainage which avoids a limited view in the depth and the need for suction. Otherwise, the prone position offers a safe alternative in view of the risk of air embolism.
To leave small amounts of pineal tissue behind in view of preservation of melatonin production has been suggested previously [33], but we usually perform a total cyst removal. We have only seen a few temporary sleep problems which were treated with a temporary melatonin substitution. Furthermore, it is not proven that a small piece of cyst will work physiologically.
Key issues on selection of patients
We assumed that the leading symptoms (headache, nausea, vomiting) of the patients are caused by a temporary increase of the intracranial pressure due to the partial aqueduct compression. During attacks of emesis, the intracranial pressure might be markedly elevated so that the compression of the aqueduct is overcome. All other potential causes for the complaints must be excluded. All patients had been thoroughly investigated prior to surgery in view of the leading symptoms (ophthalmology, neurology or neuropediatrics, internal medicine, neuropsychology, psychiatry) to rule out any other possible cause for their symptoms. We have to clearly state that the decision for resection of a simple pineal cyst without ventriculomegaly has to be taken with caution and after a long discussion with the patient and failure of all other conservative measures to control his or her symptoms.
Limitation
We could not rule out any placebo effect. It was pointed out that young female patients are more susceptible to placebo effect [51]. Most of our series were young females. However, the long-term success in most of our patients as well as the improvement in our pediatric cohort makes a placebo effect very unlikely. The patient cohort is still a subject to selection bias as most of the patients with pineal cysts and without symptoms do not seek medical attention. Furthermore, we have to state that our study is a case study (level of evidence 4 according to the Oxford Centre of Evidence-based Medicine).
Conclusion
To date, scientific information about surgical therapy of pineal cysts without hydrocephalus has been limited. We suggest that pineal cysts resection can be done also in the absence of ventriculomegaly in selected patients. The high percentage of postoperative resolution of quality of life limiting symptoms in our series demonstrates a good indication for pineal cyst resection. Preoperatively, other causes of the leading symptoms have to be excluded. Intermittent occlusion of the aqueduct may cause increased intracranial pressure leading to intermittent symptoms. Furthermore, a possible venous hypertension due to compression of the deep venous structures through the pineal cyst may also be responsible for the symptoms. However, the decision-making remains difficult since no reliable predictors are available. There is still insufficient scientific evidence to generally recommend this technique as a treatment for headache and other unspecific symptoms.
Data availability
There is availability of data and material on demand.
Code availability
Software application or custom code: not applicable.
Abbreviations
- CCOS:
-
Chicago Chiari Outcome Scale
- CISS:
-
Constructive Interference in Steady State
- CSF:
-
cerebrospinal fluid
- DRG:
-
Diagnosis Related Groups
- MSR:
-
microsurgical subtotal resection
- MTR:
-
microsurgical total resection
- PEEP:
-
positive endexspiratory pressure
- SCIT:
-
supracebellar infratentorial approach
- SS:
-
semi-sitting position
- CC:
-
concord position
References
Golzarian J, Baleriaux D, Bank WO, Matos C, Flament-Durand J (1993) Pineal cyst: normal or pathological? Neuroradiology 35:251–253
Gore PA, Gonzalez LF, Rekate HL, Nakaji P (2008) Endoscopic supracerebellar infratentorial approach for pineal cyst resection: technical case report. Neurosurgery 62(108–109):discussion 109
Mamourian AC, Towfighi J (1986) Pineal cysts: MR imaging. AJNR Am J Neuroradiol 7:1081–1086
Sawamura Y, Ikeda J, Ozawa M, Minoshima Y, Saito H, Abe H (1995) Magnetic resonance images reveal a high incidence of asymptomatic pineal cysts in young women. Neurosurgery 37(11–15):discussion 15 16
Hasegawa A, Ohtsubo K, Mori W (1987) Pineal gland in old age; quantitative and qualitative morphological study of 168 human autopsy cases. Brain Res 409:343–349
Kalani MY, Wilson DA, Koechlin NO, Abuhusain HJ, Dlouhy BJ, Gunawardena MP et al (2015) Pineal cyst resection in the absence of ventriculomegaly or Parinaud’s syndrome: clinical outcomes and implications for patient selection. J Neurosurg 123:352–356
Tapp E, Huxley M (1972) The histological appearance of the human pineal gland from puberty to old age. J Pathol 108:137–144
Chandy MJ, Damaraju SC (1998) Benign tumours of the pineal region: a prospective study from 1983 to 1997. Br J Neurosurg 12:228–233
Engel U, Gottschalk S, Niehaus L, Lehmann R, May C, Vogel S et al (2000) Cystic lesions of the pineal region—MRI and pathology. Neuroradiology 42:399–402
Fain JS, Tomlinson FH, Scheithauer BW, Parisi JE, Fletcher GP, Kelly PJ et al (1994) Symptomatic glial cysts of the pineal gland. J Neurosurg 80:454–460
Kang HS, Kim DG, Han DH (1998) Large glial cyst of the pineal gland: a possible growth mechanism Case report. J Neurosurg 88:138–140
Klein P, Rubinstein LJ (1989) Benign symptomatic glial cysts of the pineal gland: a report of seven cases and review of the literature. J Neurol Neurosurg Psychiatry 52:991–995
Mandera M, Marcol W, Bierzynska-Macyszyn G, Kluczewska E (2003) Pineal cysts in childhood. Childs Nerv Syst 19:750–755
McNeely PD, Howes WJ, Mehta V (2003) Pineal apoplexy: is it a facilitator for the development of pineal cysts? Can J Neurol Sci 30:67–71
Michielsen G, Benoit Y, Baert E, Meire F, Caemaert J (2002) Symptomatic pineal cysts: clinical manifestations and management. Acta Neurochir (Wien) 144(233–242):discussion 242
Stern JD, Ross DA (1993) Stereotactic management of benign pineal region cysts: report of two cases. Neurosurgery 32(310–314):discussion 314
Tartara F, Regolo P, Terreni MR, Giovanelli M (2000) Glial cyst of the pineal gland: case report and considerations about surgical management. J Neurosurg Sci 44:89–93
Tirakotai W, Schulte DM, Bauer BL, Bertalanffy H, Hellwig D (2004) Neuroendoscopic surgery of intracranial cysts in adults. Childs Nerv Syst 20:842–851
Turtz AR, Hughes WB, Goldman HW (1995) Endoscopic treatment of a symptomatic pineal cyst: technical case report. Neurosurgery 37(1013–1014):discussion 1014 1015
Al-Holou WN, Terman SW, Kilburg C, Garton HJ, Muraszko KM, Chandler WF et al (2011) Prevalence and natural history of pineal cysts in adults. J Neurosurg 115:1106–1114
Di Costanzo A, Tedeschi G, Di Salle F, Golia F, Morrone R, Bonavita V (1993) Pineal cysts: an incidental MRI finding? J Neurol Neurosurg Psychiatry 56:207–208
Evans RW, Peres MF (2010) Headaches and pineal cysts. Headache 50:666–668
Seifert CL, Woeller A, Valet M, Zimmer C, Berthele A, Tolle T et al (2008) Headaches and pineal cyst: a case-control study. Headache 48:448–452
Costa F, Fornari M, Valla P, Servello D (2008) Symptomatic pineal cyst: case report and review of the literature. Minim Invasive Neurosurg 51:231–233
Maurer PK, Ecklund J, Parisi JE, Ondra S (1990) Symptomatic pineal cyst: case report. Neurosurgery 27(451–453):discussion 453 454
Patel AJ, Fuller GN, Wildrick DM, Sawaya R (2005) Pineal cyst apoplexy: case report and review of the literature. Neurosurgery 57(E1066):discussion E1066
Pitskhelauri DI, Konovalov AN, Abramov IT, Danilov GV, Pronin IN, Alexandrova EV et al (2019) Pineal cyst-related aqueductal stenosis as cause of intractable headaches in nonhydrocephalic patients. World Neurosurg 123:e147–e155
Vaquero J, Martinez R, Escandon J, Bravo G (1988) Symptomatic glial cysts of the pineal gland. Surg Neurol 30:468–470
Wisoff JH, Epstein F (1992) Surgical management of symptomatic pineal cysts. J Neurosurg 77:896–900
Zhao W, Zhu DM, Zhang Y, Zhang C, Wang Y, Yang Y et al (2019) Pineal gland abnormality in major depressive disorder. Psychiatry Res Neuroimaging 289:13–17
Majovsky M, Netuka D, Benes V (2018) Is surgery for pineal cysts safe and effective? Short review. Neurosurg Rev 41:119–124
Aliaga L, Hekman KE, Yassari R, Straus D, Luther G, Chen J et al (2012) A novel scoring system for assessing Chiari malformation type I treatment outcomes. Neurosurgery 70(656–664):discussion 664 655
Majovsky M, Netuka D, Benes V (2017) Conservative and surgical treatment of patients with pineal cysts: prospective case series of 110 patients. World Neurosurg 105:199–205
Kawashima M, Rhoton AL Jr, Matsushima T (2002) Comparison of posterior approaches to the posterior incisural space: microsurgical anatomy and proposal of a new method, the occipital bi-transtentorial/falcine approach. Neurosurgery 51(1208–1220):discussion 1220 1201
Marx S, El Refaee E, Langner S, Schroeder HWS (2017) Four-hand suction-irrigation technique leads to gross total resection and long-term progression-free survival in fourth ventricular ependymoma. World Neurosurg 107:437–444
Al-Holou WN, Garton HJ, Muraszko KM, Ibrahim M, Maher CO (2009) Prevalence of pineal cysts in children and young adults Clinical article. J Neurosurg Pediatr 4:230–236
Al-Holou WN, Maher CO, Muraszko KM, Garton HJ (2010) The natural history of pineal cysts in children and young adults. J Neurosurg Pediatr 5:162–166
Choy W, Kim W, Spasic M, Voth B, Yew A, Yang I (2011) Pineal cyst: a review of clinical and radiological features. Neurosurg Clin N Am 22:341–351 (vii)
Pu Y, Mahankali S, Hou J, Li J, Lancaster JL, Gao JH et al (2007) High prevalence of pineal cysts in healthy adults demonstrated by high-resolution, noncontrast brain MR imaging. AJNR Am J Neuroradiol 28:1706–1709
Fetell MR, Bruce JN, Burke AM, Cross DT, Torres RA, Powers JM et al (1991) Non-neoplastic pineal cysts. Neurology 41:1034–1040
Oeckler R, Feiden W (1991) Benign symptomatic lesions of the pineal gland. Report of seven cases treated surgically. Acta Neurochir (Wien) 108:40–44
Osborn RE, Deen HG, Kerber CW, Glass RF (1989) A case of hemorrhagic pineal cyst: MR/CT correlation. Neuroradiology 31:187–189
El Damaty A, Fleck S, Matthes M, Baldauf J, Schroeder HWS (2019) Pineal cyst without hydrocephalus: clinical presentation and postoperative clinical course after infratentorial supracerebellar resection. World Neurosurg 129:e530–e537
Eide PK, Ringstad G (2017) Results of surgery in symptomatic non-hydrocephalic pineal cysts: role of magnetic resonance imaging biomarkers indicative of central venous hypertension. Acta Neurochir (Wien) 159:349–361
Eide PK, Ringstad G (2016) Increased pulsatile intracranial pressure in patients with symptomatic pineal cysts and magnetic resonance imaging biomarkers indicative of central venous hypertension. J Neurol Sci 367:247–255
Eide PK, Pripp AH, Ringstad GA (2016) Magnetic resonance imaging biomarkers indicate a central venous hypertension syndrome in patients with symptomatic pineal cysts. J Neurol Sci 363:207–216
Masina R, Ansaripour A, Benes V, Berhouma M, Choque-Velasquez J, Eide PK, et al 2021 Surgical treatment of symptomatic pineal cysts without hydrocephalus-meta-analysis of the published literature. Acta Neurochir(Wien)
Berhouma M, Ni H, Delabar V, Tahhan N, Memou Salem S, Mottolese C et al (2015) Update on the management of pineal cysts: case series and a review of the literature. Neurochirurgie 61:201–207
Qi S, Fan J, Zhang XA, Zhang H, Qiu B, Fang L (2014) Radical resection of nongerminomatous pineal region tumors via the occipital transtentorial approach based on arachnoidal consideration: experience on a series of 143 patients. Acta Neurochir (Wien) 156:2253–2262
Yoshimoto K, Araki Y, Amano T, Matsumoto K, Nakamizo A, Sasaki T (2013) Clinical features and pathophysiological mechanism of the hemianoptic complication after the occipital transtentorial approach. Clin Neurol Neurosurg 115:1250–1256
Weimer K, Gulewitsch MD, Schlarb AA, Schwille-Kiuntke J, Klosterhalfen S, Enck P (2013) Placebo effects in children: a review. Pediatr Res 74:96–102
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors meet all 3 of the following conditions in accordance with the “Consensus Statement on Surgery Journals Authorship—2005”:
1) Authors make substantial contributions to conception and design: Fleck, El Damaty, Schroeder, Matthes; acquisition of data: all authors; analysis and interpretation of data: Fleck, El Damaty, Lange, Matthes, Schroeder.
2) Authors participate in drafting the article or revising it critically for important intellectual content: all authors.
3) Authors give final approval of the version to be submitted and any revised version: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study complied with the Declaration of Helsinki and was approved by the ethics committee of University Medicine Greifswald. All patients gave written informed consent.
Consent for publication
The authors transfer to Springer (respective to owner if other than Springer and for US government employees: to the extent transferable) the non-exclusive publication rights and he warrants that his/her contribution is original and that he/she has full power to make this grant. The author signs for and accepts responsibility for releasing this material on behalf of any and all co-authors. This transfer of publication rights covers the non-exclusive right to reproduce and distribute the article, including reprints, translations, photographic reproductions, microform, electronic form (offline, online), or any other reproductions of similar nature.
The author may self-archive an author-created version of his article on his own website and his institution’s repository, including his final version; however, he may not use Springer’s PDF version which is posted on www.springerlink.com. Furthermore, the author may only post his version provided acknowledgement is given to the Journal and Springer as one of the original places of publication and a link is inserted to the published article on Springer’s website.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fleck, S., Damaty, A.E., Lange, I. et al. Pineal cysts without hydrocephalus: microsurgical resection via an infratentorial-supracerebellar approach—surgical strategies, complications, and their avoidance. Neurosurg Rev 45, 3327–3337 (2022). https://doi.org/10.1007/s10143-022-01831-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-022-01831-2