Abstract
Spontaneous intracerebral hemorrhage (ICH) has an increasing incidence and a worse outcome in elderly patients. The ability to predict the functional outcome in these patients can be helpful in supporting treatment decisions and establishing prognostic expectations. We evaluated the performance of a machine learning (ML) model to predict the 6-month functional status in elderly patients with ICH leveraging the predictive value of the clinical characteristics at hospital admission. Data were extracted by a retrospective multicentric database of patients ≥ 70 years of age consecutively admitted for the management of spontaneous ICH between January 1, 2014 and December 31, 2019. Relevant demographic, clinical, and radiological variables were selected by a feature selection algorithm (Boruta) and used to build a ML model. Outcome was determined according to the Glasgow Outcome Scale (GOS) at 6 months from ICH: dead (GOS 1), poor outcome (GOS 2–3: vegetative status/severe disability), and good outcome (GOS 4–5: moderate disability/good recovery). Ten features were selected by Boruta with the following relative importance order in the ML model: Glasgow Coma Scale, Charlson Comorbidity Index, ICH score, ICH volume, pupillary status, brainstem location, age, anticoagulant/antiplatelet agents, intraventricular hemorrhage, and cerebellar location. Random forest prediction model, evaluated on the hold-out test set, achieved an AUC of 0.96 (0.94–0.98), 0.89 (0.86–0.93), and 0.93 (0.90–0.95) for dead, poor, and good outcome classes, respectively, demonstrating high discriminative ability. A random forest classifier was successfully trained and internally validated to stratify elderly patients with spontaneous ICH into prognostic subclasses. The predictive value is enhanced by the ability of ML model to identify synergy among variables.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous intracerebral hemorrhage (ICH) has an estimated annual incidence of 25/100,000 persons and an overall 12-month mortality of 40–60%, mainly within the first month [2, 21]. The best ICH management remains conservative, while surgery appears controversial and mainly advocated for superficial hemorrhage in younger patients [9, 21, 36].
ICH incidence increases with age, posing a higher assistance burden in countries with aging population [2, 15, 23]. However, besides age, a large number of factors need to be considered for risk stratification, including comorbidities, drugs, ICH volume and site, and neurological status [15].
Functional status rather than mortality should be considered as the correct outcome measurement after ICH, especially in older patients with increasing frailty and reduced long-term expectations. Predicting functional outcome in these patients can be helpful in supporting treatment decisions and prognostic expectations.
Recently, machine learning (ML) has emerged as a powerful tool to develop predictive algorithms from a large amount of data [35].
Here, we built a ML model to predict the 6-month functional status in elderly patients with ICH leveraging the predictive value of the clinical characteristics at hospital admission.
Methods
Patient population
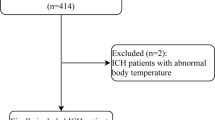
We included patients with age ≥ 70 years consecutively admitted at the emergency department of 3 Italian tertiary referral cerebrovascular centers for spontaneous ICH between January 2014 and December 2019.
All the participating hospitals had a 24/7 A&E service and a territory reference stroke unit with an average population above 500,000 persons.
Patients with trauma, ruptured vascular malformations, or hemorrhagic neoplasms were excluded.
Management consisted of surgical evacuation or medical treatment with hyperosmolar solutions, steroids, antiepileptics, head elevation, sedation, and ventilatory and cardiovascular support.
Ethical approval was waived by the local committee due to the retrospective and anonymous nature of the study.
Data collection
We collected demographical, clinical, and neuroradiological data: patients’ age and gender, comorbidities, current medications, Charlson Comorbidity Index (CCI), and smoking habit; Glasgow Coma Scale (GCS), pupillary size and light reaction, neurological deficits, and seizures at admission; ICH side, location (including lobar, basal ganglia, brainstem, or posterior cranial fossa) and volume measured by the ABC/2 method, and presence of intraventricular hemorrhage; data about surgical or medical treatment; length of hospital stay; and Glasgow Outcome Scale (GOS) at discharge and 6-month follow-up.
Outcome measures
We identified 3 outcome groups according to 6-month GOS after ICH: dead (D, GOS-1); poor outcome: vegetative status (VS, GOS-2) and severe disability (SD, GOS-3); and good outcome: moderate disability (MD, GOS-4) and good recovery (GR, GOS-5).
We evaluated a ML model feasibility and performance to predict 6-month GOS leveraging the predictive value of patient’s characteristics at admission.
The Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) statement guidelines were followed to minimize the bias risk during the development phase and correctly validate the predictive ability of our ML models during the testing phase [31].
Exploratory data analysis
The association between patients’ variables and outcome was investigated with multiple tests (chi-square test, Student’s t test, ANOVA, Mann–Whitney U test, and Fisher’s exact test). Alfa was set at 0.05, and Holm-Bonferroni correction was applied to shield against type 1 error in the setting of multiple comparisons. All covariates reporting a p value < 0.05 at the univariate inferential analysis were further investigated with a multivariable logistic regression model.
Machine learning model development
The architecture adopted for ML pipeline is outlined in Fig. 1. Considering the characteristics of our dataset, sample size, and outcomes, a random forest (RF) model was deemed the most appropriate ML model [6]. RF is a popular ensemble ML algorithm with easy hyperparameter tuning, lower risk of bias compared to a simple decision tree, elevated generalizability and accuracy, ability to capture non-linear data patterns, and applicability to different data volumes. It works by fitting a number of different decision trees (ensemble): majority “voting”/ “averaging” of all trees’ outcomes are used to classify/predict each patient’s outcome, thus improving predictive accuracy and controlling over-fitting.
Then, the patients’ cohort was randomly split into a training set and a hold-out test set following an 80:20 ratio. No data from the hold-out test set was ever employed during feature selection, synthetic minority over-sampling, and training phase.
Feature selection
RF algorithm can achieve reliable performances even when the number of features exceeds that of the dataset instances or when most features are irrelevant to the outcome. Feature selection was performed using Boruta (v.0.3) [26], an all-relevant feature selection algorithm wrapped around RF allowing to identify and retain only the most predictive patient’s variables (input features). Reducing input features shows several advantages: (a) the model’s degrees of freedom reduction decreases the over-fitting and improves the generalizability, and (b) the emphasis of the most important variables enhances the model’s interpretability.
Synthetic minority over-sampling technique
Issues deriving from the imbalanced nature of our dataset were explored. When training with imbalanced data, ML algorithms preferentially learnt from the majority than the minority class, thus resulting in limitedly generalizable predictive models. Thus, the original training dataset was balanced using the synthetic minority over-sampling technique-nominal continuous (SMOTE-NC) [8].
Random forest training and hyperparameter tuning
The hyperparameter optimization or tuning consists in finding the optimal hyperparameters set for a ML algorithm, which are used to control the learning process. A fivefold cross validation search grid was used on the training set for hyperparameter tuning of RF model. The best performing hyperparameters (e.g., number of estimators, learning rate, max depth) and their spaces tuned via a search grid are reported in the Supplementary Material.
Random forest performance metrics evaluation
Finally, the optimized RF model works stratifying hold-out test set patients into prognostic subclasses predicting the 6-month status according to the 3 GOS groups reported above. RF model performance was thoroughly evaluated considering the following metrics:
-
Area under the receiving operative characteristics (AUC-ROC)
-
Accuracy
-
Positive predictive value (PPV) or precision
-
Sensitivity or recall
-
Specificity
-
Negative predictive value (NPV)
-
False positive rate (FPR)
-
F1 score
A One-vs-the-Rest (OvR) multiclass strategy was employed to extract performance metrics, then the average value and its 95% bootstrap confidence interval were computed.
Software
All statistical analyses were performed in Jupyter Notebook, using Python v.3.8.2 whose packages included the following: “Scikit-learn” to develop and train the random forest models, “Numpy” for Excel dataset handling, “imbalanced-learn” to solve class imbalance problem, “Sci-py” to perform univariable statistical association tests, “Statsmodels” to perform multivariable analyses, “Boruta” to perform recursive feature selection, and “LIME” v.0.2.0.1 to interpret the ML model. The entire source code utilized to develop the RF model is available at https://github.com/valerio-mc/ML-in-ICH.
Results
Patient population
In this multicenter series, 809 consecutive ICH patients were admitted in a 5-year period (Table 1). Mean age was 79.85 ± 6.35 years, and 389 out of 809 (48.0%) were men. Mean GCS score at admission was 10.43 ± 4.12, while mean ICH score was 1.96 ± 1.5. The mean number of comorbidities was 2.6 ± 1.61 with the most frequents being hypertension (82%), cardiovascular diseases (42%), and diabetes (21%; Supplementary Table 1). The mean number of medications per patient was 3.47 ± 1.98; among them were antihypertensives (72%), antiplatelets (40%), and anticoagulants (21%). Noteworthy, 494 patients (61%) were under anticoagulants and/or antiplatelets. The most frequently ICH topographies were parietal lobes (329 patients, 41%) and cerebellum (99–13%). Only a minority of patients (39/809; 4.8%), generally the youngest (mean 74 years), underwent surgical evacuation.
At 6-month follow-up, 301 patients (37.2%) were dead, 261 (32.3%) had poor outcome (SD-VS), and 247 (30.5%) had good outcome (MD-GR).
Univariate analysis
At univariate analysis, 20 variables showed a significant association with the 6-month GOS after ICH (Table 1). Mean age, hematoma volume, ICH score, number of drugs, anticoagulants/antiplatelets, comorbidities, and CCI were higher in patients who died at 6 months conversely than mean GCS that was lower. A higher incidence of intraventricular hemorrhage (IVH), subarachnoid hemorrhage (SAH), non-isochoric pupils, and antiplatelet and antacid prescription was reported for patients who died at 6 months. The presence of renal insufficiency and neurological comorbidities was also associated with poor 6-month outcome (GOS 2–3). ICHs involving frontal lobe, temporal lobe, or brainstem were more frequently seen in patients with poorer 6-month outcome.
Random forest performance metrics evaluation
Features selected for RF classifier via Boruta were age, IVH, hematoma volume, GCS at admission, ICH score, isochoric pupils at admission, anticoagulant/antiplatelet, Charlson Comorbidity Index, and brainstem or cerebellum involvement.
RF prediction model, evaluated on the hold-out test set, achieved an AUC of 0.96 (0.94–0.98), 0.89 (0.86–0.93), and 0.93 (0.90–0.95) for dead, poor, and good outcome classes, respectively, demonstrating high discriminative ability (Fig. 2). Moderate to high reliability was reported across all performance metrics for all prognostic outcome classes. Evaluation of the model on the hold-out test set corresponds to internal validation, providing reliable expectation on the model’s performance on new external data (Table 2).
Multivariate analysis
At multivariate analysis, several patient variables were significantly associated with 6-month functional status. Results are summarized in Table 3.
Briefly, older age, higher ICH score, and larger ICH volume were associated with the risk of death at 6 months, while cerebellar location and higher GCS at admission were associated with the lower risk of death at 6 months.
Brainstem involvement, older age, higher CCI, and larger hematoma volume were associated with an increased risk of poor 6-month outcome (GOS 2–3). Conversely, higher GCS decreased the risk of poor 6-month outcome.
Model interpretation
Importance plot of relative features for the RF model is reported in Fig. 2. The parameters with strongest predictive values were as follows: GCS at admission, Charlson Comorbidity Index, ICH score, hematoma volume, pupillary status, and brainstem involvement.
We further introduced a locally interpretable model-agnostic explanations (LIME) algorithm to quantify the feature contribution and polarity for each patient, thus providing an interpretable relationship between patient’s characteristics and RF model prediction. An example is illustrated in Fig. 3. Understanding the reason behind both correct and incorrect model predictions can increase clinicians’ trust in model behavior and performance.
Output of local interpretable model–agnostic explanation (LIME) with random forest classifiers applied to one correctly predicted patient that died within 6 months. The figure reveals the role of various features in influencing the outcome prediction for each patient. A Patient’s characteristics. B Features contributions on predicted probabilities (red, risk factor; blue, protective factor). C Predicted probability of death at 6 months. IVH, intraventricular hemorrhage; GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage
Discussion
ICH shows increasing incidence and dismal outcome in elderly population [2, 15, 23]. This is confirmed by our multicenter series showing that about 2/3 of patients died or were in poor functional status after 6 months from ICH presentation. The optimal treatment is still debated for aged patients as, for this population, it is more difficult to determine the prognostic criteria guiding therapeutic choices due to the numerous variables related to their multiple morbidity. For the same reason and the reduced potential of recovery, older patients are also generally poor candidates for surgery [9, 21, 36].
Current treatment algorithms are based on the correction of risk factors to prevent re-bleeding such as a hypocoagulative status and arterial hypertension and on the reduction of secondary damage due to edema and intracranial hypertension [21]. In this scenario, the ability to predict the functional outcome of elderly patients could provide a strong support to the decision-making process regarding management and communication with family.
Conventional statistics
In this study, we investigated the role of 114 different variables including, among the others, the following: age, drugs, comorbidities, neurological status, as well as some radiological features such as ICH side, location, and volume. Several of these were significantly associated with death or poor 6-month functional outcome at univariate analysis.
Regarding the risk of death, we divided these factors in subject-related (age, number of drugs including antithrombotics, number of comorbidities, and CCI), ICH-related (volume, IVH, SAH), and presenting clinical status–related (GCS, non-isochoric pupils).
Some specific comorbidities such as renal insufficiency and neurological disorders as well as some ICH topographies (frontal, temporal, brainstem) instead appeared related to poor outcome.
At multivariate analysis (Table 3), increasing age, larger hematoma volume, lower GCS at admission, ICH score, and brainstem location were significantly associated with death, poor outcome, or both.
Conversely, cerebellar ICH location was associated with a better clinical outcome at univariate analysis and to a reduced risk of death at multivariate analysis.
Machine learning model: variable selection and relative importance
A ML model allows to elaborate a prediction algorithm including a number of variables hardly manageable with conventional statistics [22]. In fact, ML also explores non-linear correlations among variables and detects not only significant associations with outcomes, but also the synergy among variables in outcome prediction. Indeed, an electronic health record–based prediction model has been shown to be more accurate in predicting the risk of adverse outcome than traditional models using the a priori selected clinical variables and predictors representing a source of agnostic assessment that is independent of practitioner experience and provide additional assurance to families when considering ongoing intervention [42]. Moreover, the relative weight of single variables may vary among patients due to the interaction and interplay with the others. For example, the use of medications may play different weights among patients’ prognostication according to other features such as age and comorbidities. This allows discovering an unexpected association between variables and outcomes, confirming the role of known variables and emphasizing their combination.
As shown in Fig. 2D, the relative importance of the 10 variables selected by Boruta for our RF model differs with the following order:
-
1)
GCS: presenting GCS shows the higher relative importance in our model. More than 20% of patients have a pre-hospital GCS deterioration (drop of 2 or more GCS points) [30] and another 20% presenting with a GCS score ≥ 13 have an early deterioration after hospital admission [13]. Moreover, elderly patients usually present a worse GCS than younger ones [15]. Baseline GCS and GCS deterioration were found significantly associated with ICH-related mortality and poor functional outcome in several studies [1, 38].
-
2)
CCI: our study confirms previous findings that comorbidities as measured by the CCI independently influence the outcome after ICH [4, 24]. Interestingly, CCI showed a higher relative importance than hematoma volume and patient’s age in our model.
-
3)
ICH score: it is a 6-point score (0-to-5) developed in 2001 to predict the 30-day mortality after ICH [19]. It includes GCS (3–4/5–12/13–15), ICH volume (< 30 ml or > 30 ml), IVH, infratentorial location, and an age threshold of 80 years. This score has also been related to functional outcome in ICH patients [11, 12, 20]. Noticeably, all ICH score variables were automatically selected by our RF model as significant for 6-month outcome prediction. However, some authors observed that the current survival of poor ICH score grades is higher than what prognosticated at the time of score developed 20 years ago [29]. Unlike ICH score using categorical data, the RF model adopts continuous variables as age, ICH volume, and GCS to depict their importance in single patients. The selection of ICH score as feature reinforces the role of other variables.
-
4)
ICH volume: the prognosticating value of ICH volume has been shown as dependent by the topography of the hematoma. Indeed, supratentorial ICH ≥ 30 ml, thalamic ICH ≥ 8 ml, basal ganglia ICH ≥ 18 ml, and cerebellar ICH ≥ 15 ml are cutoff volumes for poor outcome [7, 25, 27]. These differences were not taken into account by the original ICH score.
-
5)
Pupillary status: it is a reliable sign of cerebral herniation and intracranial hypertension. ICH volume and midline shift were correlated with impaired pupillary reactivity [28].
-
6)
Brainstem location: despite often classified as “infratentorial” as well as the cerebellar location, brainstem ICH shows worse clinical presentation and poorer outcome [10, 32].
-
7)
Age: elderly is a risk factor for ICH occurrence and worse outcome [2, 15, 23]. However, it is difficult to distinguish between age itself and associated comorbidities [1]. In our model, the weight of CCI appeared higher than age for outcome prognostication. Indeed, as already shown in several studies on intracranial traumatic and non-traumatic ICH, age is one among, but not the most important risk factor for outcome prediction [3, 37]. In our cohort of elderly patients, the RF model detected an age threshold of 80 years for poor outcome (Fig. 3BB).
-
8)
Anticoagulant/antiplatelet agents: these drugs are associated with an increased risk of ICH occurrence and poor outcome [34, 41]. Despite diverse bleeding odds have been reported with different medications, only antiplatelets showed a significant association with outcome at univariate analysis (Table 1 and Supplementary Table 1), but neither at multivariate statistics nor at RF model. Noticeable, in all participating centers, there was the attitude to pharmacologically reverse the coagulative status, when possible, immediately after the radiological evidence of ICH. However, our ML model automatically selected as significant for outcome prognostication the entire category of anticoagulant/antiplatelet agents.
-
9)
IVH: intraventricular bleeding is more common after thalamic bleeding [27]. The association between IVH and hyperpyrexia and, in turn, their negative influence on prognosis of ICH patients is already known [16, 33].
-
10)
Cerebellar location: cerebellar ICH appeared associated to a better outcome with both conventional statistics and ML mode in agreement with several previous studies [10, 25, 32]. In fact, current American and European guidelines suggest a more aggressive surgical attitude in cases of cerebellar ICH > 3 cm in diameter (about 14 ml) [21, 36].
Machine learning model: RF model accuracy and clinical implication
We built a robust RF prediction model with high discriminative ability for death (GOS 1), poor outcome (GOS 2–3), and good outcome (GOS 4–5). RF metrics appeared particularly performing in prediction of death (about 90% of accuracy) and both poor and good outcomes (accuracy of 82% and 84%, respectively; Table 2). F1 score, which is a balance between precision and recall, was less reliable for poor outcome detection, due to a relatively high number of false negatives with a recall of 65%. This was balanced by the more satisfactory F1 metrics for the other two outcomes. RF metrics showed that our model has a slightly “optimistic” predictive attitude, with an increasing FPR at improving functional status, suggesting caution in over-emphasizing a good outcome prediction.
Previous evidences and current novelties
Other authors previously reported their experiences with ML for ICH prognostication.
Wang et al. [39] found that among 39 ML methods, RF was the most accurate for predicting 1-month and 6-month functional outcomes after ICH in a younger cohort of patients.
Similarly, Hall and colleagues [17] demonstrated that hematoma volume, its expansion, GCS score, age, and IVH were the most important variables associated with for outcome prediction at 14-day and 3-month using decision tree and RF models.
Baseline hematoma volume (> 20 ml), IVH, age (> 53 years), and diabetes had the highest percentage of influence weight in a ML model developed by He and colleagues [18] to estimate poor outcome in supratentorial spontaneous ICH treated with conservative treatment. Another ML-based radiomics-clinical model constructed to predict IVH growth after spontaneous ICH found as independent predictors of IVH growth: hypercholesterolemia, baseline Graeb score, time to initial CT, international normalized ratio, and Rad score [43].
Fernandez-Lozano et al. [14] also confirmed that the RF model was the most accurate ML approach, and neurological status during the first 48 h, axillary temperature, early neurological deterioration, leukocyte count, and blood glucose were the most relevant outcome predictors in a large series of patients with ischemic strokes and ICH.
However, all these previous studies focused on a general, unselected, population including younger people and overall agreeing that prognostication in ICH patients is difficult and age is the main functional outcome predictor.
In our study, instead, firstly, we focused the analysis on the elderly population, which is the most often affected by spontaneous ICH and the most problematic for the decision-making process about treatment due to the elevated risk of expected unfunctional outcome.
Our results support the clinicians in defining the expected outcome for ICH patients aged above 70 years.
Study limitations
Our study has several limitations: first, it has a retrospective nature and limited differences among treatments. Indeed, only a minority of patients underwent ICH evacuation, while the majority had medical treatment, generally based on osmotic agents or hypertonic saline solution at different doses among the participating centers. This made a direct association between treatment and outcome difficult to assess, but assuming that all the cases received the best medical treatment and an eventual evacuation when the clinical status had requested, we believe the results of the present study may reinforce the awareness that some independent variables may play a major role in outcome prediction. In fact, we built a very accurate model able to detect numerous major and minor potential variables, weighting their role not only in dead/alive status prediction, but, above all, also in the 6-month functional status prognostication.
Perspectives
The input of selected features allows to obtain a predictive probability of the outcome of interest, also allowing to visualize the weight of each variable for single patient prognostication (Fig. 3). This model, available on a freely accessible repository a simple and user-friendly webpage with an interface (3A), could be validated and implemented by external research groups, allowing clinicians to insert details of the 10 selected features of each patient and to calculate the predictive probability for the three main outcomes (dead, poor, and good) at 6 months.
Although the power of prognostication is an undoubtedly important tool helping to assess the severity of illness and provide information to families, the experience and sensitivity of the expert clinicians using these fallible instruments should never be put aside to avoid the generation of a “self-fulfilling prophecy” attitude discouraging from the effort to maximize the treatment opportunities for patients, which is the aim of this study [5, 29, 40].
Conclusions
A RF classifier was successfully trained and internally validated to stratify elderly patients with spontaneous ICH into prognostic subclasses based on GOS. The predictive value is enhanced by the ability of the ML model to identify factors not relevant if analyzed singularly, but becoming significant when combined each other in a real-life setting.
Availability of data and material
Available upon reasonable request.
Code availability
Not applicable.
Change history
24 August 2022
Missing Open Access funding information has been added in the Funding Note.
References
Al-Mufti F, Thabet AM, Singh T, El-Ghanem M, Amuluru K, Gandhi CD (2018) Clinical and radiographic predictors of intracerebral hemorrhage outcome. Intervent Neurol 7:118–136. https://doi.org/10.1159/000484571
van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ (2010) Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 9:167–176. https://doi.org/10.1016/S1474-4422(09)70340-0
Bagg S, Pombo AP, Hopman W (2002) Effect of age on functional outcomes after stroke rehabilitation. Stroke 33:179–185. https://doi.org/10.1161/hs0102.101224
Bar B, Hemphill JC (2011) Charlson Comorbidity Index adjustment in intracerebral hemorrhage. Stroke 42:2944–2946. https://doi.org/10.1161/STROKEAHA.111.617639
Becker KJ, Baxter AB, Cohen WA, Bybee HM, Tirschwell DL, Newell DW, Winn HR, Longstreth WT (2001) Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology 56:766–772. https://doi.org/10.1212/WNL.56.6.766
Breiman L (2001) Random forests. Mach Learn 45:5–32. https://doi.org/10.1023/A:1010933404324
Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G (1993) Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 24:987–993. https://doi.org/10.1161/01.STR.24.7.987
Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP (2002) SMOTE: Synthetic Minority Over-sampling Technique. J Artif Intell Res 16:321–357. https://doi.org/10.1613/jair.953
Chen C-J, Ding D, Ironside N, Buell TJ, Southerland AM, Woo D, Worrall BB (2019) Predictors of surgical intervention in patients with spontaneous intracerebral hemorrhage. World Neurosurgery 123:e700–e708. https://doi.org/10.1016/j.wneu.2018.11.260
Chen R, Wang X, Anderson CS, Robinson T, Lavados PM, Lindley RI, Chalmers J, Delcourt C, for the INTERACT Investigators (2019) Infratentorial intracerebral hemorrhage: relation of location to outcome. Stroke 50:1257–1259.https://doi.org/10.1161/STROKEAHA.118.023766
Cheung RTF, Zou L-Y (2003) Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke 34:1717–1722. https://doi.org/10.1161/01.STR.0000078657.22835.B9
Clarke JL, Johnston SC, Farrant M, Bernstein R, Tong D, Hemphill JC (2004) External validation of the ICH score. NCC 1:53–60. https://doi.org/10.1385/NCC:1:1:53
Fan J-S, Huang H-H, Chen Y-C, Yen DH-T, Kao W-F, Huang M-S, Huang C-I, Lee C-H (2012) Emergency department neurologic deterioration in patients with spontaneous intracerebral hemorrhage: incidence, predictors, and prognostic significance. Acad Emerg Med 19:133–138. https://doi.org/10.1111/j.1553-2712.2011.01285.x
Fernandez-Lozano C, Hervella P, Mato-Abad V, Rodríguez-Yáñez M, Suárez-Garaboa S, López-Dequidt I, Estany-Gestal A, Sobrino T, Campos F, Castillo J, Rodríguez-Yáñez S, Iglesias-Rey R (2021) Random forest-based prediction of stroke outcome. Sci Rep 11:10071. https://doi.org/10.1038/s41598-021-89434-7
Forti P, Maioli F, Domenico Spampinato M, Barbara C, Nativio V, Coveri M, Zoli M, Simonetti L, Di Pasquale G, Procaccianti G (2016) The effect of age on characteristics and mortality of intracerebral hemorrhage in the oldest-old. Cerebrovasc Dis 42:485–492. https://doi.org/10.1159/000448813
Hajat C, Hajat S, Sharma P (2000) Effects of poststroke pyrexia on stroke outcome: a meta-analysis of studies in patients. Stroke 31:410–414. https://doi.org/10.1161/01.STR.31.2.410
Hall AN, Weaver B, Liotta E, Maas MB, Faigle R, Mroczek DK, Naidech AM (2021) Identifying modifiable predictors of patient outcomes after intracerebral hemorrhage with machine learning. Neurocrit Care 34:73–84. https://doi.org/10.1007/s12028-020-00982-8
He X, Chen M, Du C, Zhao K, Yang M, Ma Q (2020) A novel model for predicting the outcome of intracerebral hemorrhage: based on 1186 patients. J Stroke Cerebrovasc Dis 29:104867. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.104867
Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC (2001) The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 32:891–897. https://doi.org/10.1161/01.STR.32.4.891
Hemphill JC, Farrant M, Neill TA (2009) Prospective validation of the ICH score for 12-month functional outcome. Neurology 73:1088–1094. https://doi.org/10.1212/WNL.0b013e3181b8b332
Hemphill JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D (2015) Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46:2032–2060. https://doi.org/10.1161/STR.0000000000000069
Heo J, Yoon JG, Park H, Kim YD, Nam HS, Heo JH (2019) Machine learning–based model for prediction of outcomes in acute stroke. Stroke 50:1263–1265. https://doi.org/10.1161/STROKEAHA.118.024293
Inoue Y, Miyashita F, Minematsu K, Toyoda K (2018) Clinical characteristics and outcomes of intracerebral hemorrhage in very elderly. J Stroke Cerebrovasc Dis 27:97–102. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.08.006
Jiménez Caballero PE, López Espuela F, Portilla Cuenca JC, Ramírez Moreno JM, Pedrera Zamorano JD, Casado Naranjo I (2013) Charlson Comorbidity Index in ischemic stroke and intracerebral hemorrhage as predictor of mortality and functional outcome after 6 months. J Stroke Cerebrovasc Dis 22:e214–e218. https://doi.org/10.1016/j.jstrokecerebrovasdis.2012.11.014
Kuramatsu JB, Biffi A, Gerner ST, Sembill JA, Sprügel MI, Leasure A, Sansing L, Matouk C, Falcone GJ, Endres M, Haeusler KG, Sobesky J, Schurig J, Zweynert S, Bauer M, Vajkoczy P, Ringleb PA, Purrucker J, Rizos T, Volkmann J, Müllges W, Kraft P, Schubert A-L, Erbguth F, Nueckel M, Schellinger PD, Glahn J, Knappe UJ, Fink GR, Dohmen C, Stetefeld H, Fisse AL, Minnerup J, Hagemann G, Rakers F, Reichmann H, Schneider H, Rahmig J, Ludolph AC, Stösser S, Neugebauer H, Röther J, Michels P, Schwarz M, Reimann G, Bäzner H, Schwert H, Claßen J, Michalski D, Grau A, Palm F, Urbanek C, Wöhrle JC, Alshammari F, Horn M, Bahner D, Witte OW, Günther A, Hamann GF, Hagen M, Roeder SS, Lücking H, Dörfler A, Testai FD, Woo D, Schwab S, Sheth KN, Huttner HB (2019) Association of surgical hematoma evacuation vs conservative treatment with functional outcome in patients with cerebellar intracerebral hemorrhage. JAMA 322:1392. https://doi.org/10.1001/jama.2019.13014
Kursa MB, Rudnicki WR (2010) Feature selection with the Boruta package. J Stat Softw 36:1–13. https://doi.org/10.18637/jss.v036.i11
Leasure AC, Sheth KN, Comeau M, Aldridge C, Worrall BB, Vashkevich A, Rosand J, Langefeld C, Moomaw CJ, Woo D, Falcone GJ (2019) Identification and validation of hematoma volume cutoffs in spontaneous, supratentorial deep intracerebral hemorrhage. Stroke 50:2044–2049. https://doi.org/10.1161/STROKEAHA.118.023851
Mazhar K, Olson DM, Atem FD, Stutzman SE, Moreno J, Venkatachalam A, Aiyagari V (2021) Supratentorial intracerebral hemorrhage volume and other CT variables predict the neurological pupil index. Clin Neurol Neurosurg 200:106410. https://doi.org/10.1016/j.clineuro.2020.106410
McCracken DJ, Lovasik BP, McCracken CE, Frerich JM, McDougal ME, Ratcliff JJ, Barrow DL, Pradilla G (2019) The intracerebral hemorrhage score: a self-fulfilling prophecy? Neurosurgery 84:741–748. https://doi.org/10.1093/neuros/nyy193
Moon J-S, Janjua N, Ahmed S, Kirmani JF, Harris-Lane P, Jacob M, Ezzeddine MA, Qureshi AI (2008) Prehospital neurologic deterioration in patients with intracerebral hemorrhage. Crit Care Med 36:172–175. https://doi.org/10.1097/01.CCM.0000297876.62464.6B
Moons KGM, Altman DG, Reitsma JB, Ioannidis JPA, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS (2015) Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 162:W1–W73. https://doi.org/10.7326/M14-0698
Patel VD, Garcia RM, Swor DE, Liotta EM, Maas MB, Naidech A (2020) Natural history of infratentorial intracerebral hemorrhages: two subgroups with distinct presentations and outcomes. J Stroke Cerebrovasc Dis 29:104920. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.104920
Schwarz S, Hafner K, Aschoff A, Schwab S (2000) Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology 54:354–354. https://doi.org/10.1212/WNL.54.2.354
Seiffge DJ, Goeldlin MB, Tatlisumak T, Lyrer P, Fischer U, Engelter ST, Werring DJ (2019) Meta-analysis of haematoma volume, haematoma expansion and mortality in intracerebral haemorrhage associated with oral anticoagulant use. J Neurol 266:3126–3135. https://doi.org/10.1007/s00415-019-09536-1
Senders JT, Staples PC, Karhade AV, Zaki MM, Gormley WB, Broekman MLD, Smith TR, Arnaout O (2018) Machine learning and neurosurgical outcome prediction: a systematic review. World Neurosurgery 109:476-486.e1. https://doi.org/10.1016/j.wneu.2017.09.149
Steiner T, Salman RA-S, Beer R, Christensen H, Cordonnier C, Csiba L, Forsting M, Harnof S, Klijn CJM, Krieger D, Mendelow AD, Molina C, Montaner J, Overgaard K, Petersson J, Roine RO, Schmutzhard E, Schwerdtfeger K, Stapf C, Tatlisumak T, Thomas BM, Toni D, Unterberg A, Wagner M (2014) European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 9:840–855. https://doi.org/10.1111/ijs.12309
Trevisi G, Sturiale CL, Scerrati A, Rustemi O, Ricciardi L, Raneri F, Tomatis A, Piazza A, Auricchio AM, Stifano V, Romano C, Bonis PD, Mangiola A (2020) Acute subdural hematoma in the elderly: outcome analysis in a retrospective multicentric series of 213 patients. Neurosurg Focus 10. https://doi.org/10.3171/2020.7.FOCUS20437
Wang C-W, Liu Y-J, Lee Y-H, Hueng D-Y, Fan H-C, Yang F-C, Hsueh C-J, Kao H-W, Juan C-J, Hsu H-H (2014) Hematoma shape, hematoma size, Glasgow Coma Scale score and ICH score: which predicts the 30-day mortality better for intracerebral hematoma? PLoS ONE 9:e102326. https://doi.org/10.1371/journal.pone.0102326
Wang H-L, Hsu W-Y, Lee M-H, Weng H-H, Chang S-W, Yang J-T, Tsai Y-H (2019) Automatic machine-learning-based outcome prediction in patients with primary intracerebral hemorrhage. Front Neurol 10:910. https://doi.org/10.3389/fneur.2019.00910
Witsch J, Siegerink B, Nolte CH, Sprügel M, Steiner T, Endres M, Huttner HB (2021) Prognostication after intracerebral hemorrhage: a review. Neurol Res Pract 3:22. https://doi.org/10.1186/s42466-021-00120-5
Wu Y, Zhang D, Chen H, Liu B, Zhou C (2021) Effects of prior antiplatelet therapy on mortality, functional outcome, and hematoma expansion in intracerebral hemorrhage: an updated systematic review and meta-analysis of cohort studies. Front Neurol 12:691357. https://doi.org/10.3389/fneur.2021.691357
Yu D, Williams GW, Aguilar D, Yamal J, Maroufy V, Wang X, Zhang C, Huang Y, Gu Y, Talebi Y, Wu H (2020) Machine learning prediction of the adverse outcome for nontraumatic subarachnoid hemorrhage patients. Ann Clin Transl Neurol 7:2178–2185. https://doi.org/10.1002/acn3.51208
Zhu D-Q, Chen Q, Xiang Y-L, Zhan C-Y, Zhang M-Y, Chen C, Zhuge Q-C, Chen W-J, Yang X-M, Yang Y-J (2021) Predicting intraventricular hemorrhage growth with a machine learning-based, radiomics-clinical model. Aging 13:12833–12848. https://doi.org/10.18632/aging.202954
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Gianluca Trevisi: conceptualization (equal), data curation (equal), methodology (equal), formal analysis (supportive), visualization (supportive), writing of the original draft (lead), and writing including review and editing (equal).
Valerio Maria Caccavella: methodology (equal), formal analysis (lead), software (lead), validation (lead), visualization (lead), writing of the original draft (supportive), and writing including review and editing (equal).
Alba Scerrati: data curation (equal), methodology (equal), and writing including review and editing (equal).
Francesco Signorelli: data curation (equal), methodology (equal), and writing including review and editing (equal).
Giuseppe Giovanni Salamone: data curation (equal) and writing including review and editing (equal).
Klizia Orsini: data curation (equal) and writing including review and editing (equal).
Christian Fasciani: data curation (equal) and writing including review and editing (equal).
Sonia D’Arrigo: data curation (equal) and writing including review and editing (equal).
Anna Maria Auricchio: data curation (equal) and writing including review and editing (equal).
Ginevra D’Onofrio: data curation (equal) and writing including review and editing (equal).
Francesco Salomi: data curation (equal) and writing including review and editing (equal).
Alessio Albanese: supervision (equal) and writing including review and editing (equal).
Pasquale De Bonis: supervision (equal) and writing including review and editing (equal).
Annunziato Mangiola: supervision (equal) and writing including review and editing (equal).
Carmelo Lucio Sturiale: conceptualization (equal), methodology (equal), formal analysis (supportive), writing of the original draft (supportive), and writing including review and editing (equal).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
IRB approval was not required for the retrospective collection of anonymous data. Informed consent was provided by every patient participating in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trevisi, G., Caccavella, V.M., Scerrati, A. et al. Machine learning model prediction of 6-month functional outcome in elderly patients with intracerebral hemorrhage. Neurosurg Rev 45, 2857–2867 (2022). https://doi.org/10.1007/s10143-022-01802-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-022-01802-7