Abstract
Delirium is a frequent occurring complication in surgical patients. Nevertheless, a scientific work-up of the clinical relevance of delirium after intracranial surgery is lacking. We conducted a systematic review (CRD42020166656) to evaluate the current diagnostic work-up, incidence, risk factors and health outcomes of delirium in this population. Five databases (Embase, Medline, Web of Science, PsycINFO, Cochrane Central) were searched from inception through March 31st, 2021. Twenty-four studies (5589 patients) were included for qualitative analysis and twenty-one studies for quantitative analysis (5083 patients). Validated delirium screening tools were used in 70% of the studies, consisting of the Confusion Assessment Method (intensive care unit) (45%), Delirium Observation Screening Scale (5%), Intensive Care Delirium Screening Checklist (10%), Neelon and Champagne Confusion Scale (5%) and Nursing Delirium Screening Scale (5%). Incidence of post-operative delirium after intracranial surgery was 19%, ranging from 12 to 26% caused by variation in clinical features and delirium assessment methods. Meta-regression for age and gender did not show a correlation with delirium. We present an overview of risk factors and health outcomes associated with the onset of delirium. Our review highlights the need of future research on delirium in neurosurgery, which should focus on optimizing diagnosis and assessing prognostic significance and management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Delirium is characterized by a temporary decline in the patient’s mental status affecting attention, awareness, cognition, language and/or visuospatial ability, [1] caused by dysregulation of neuronal activity [2]. Intracranial surgery evokes a parenchymal inflammatory reaction resulting in oxidative stress, which is subsequently aggravated by impaired oxygenation of the surrounding tissue due to the formation of oedema. Hypotheses describing the pathophysiology of delirium include neuro-inflammatory and oxidative reactions within the brain. Considering this, neurosurgical patients are vulnerable to delirium [2].
Unfortunately, delirium in the neurosurgical population has been under-investigated. This may be explained by the lack of consensus on definition and challenge with respect to its diagnosis [3,4,5]. Therefore, reported incidences vary, especially in case of hypoactive delirium 6]. Delirium is considered a severe complication in other populations, being a traumatic experience for patients and contributing to prolonged hospital stay, higher risk for re-operation, mortality and cognitive decline [7,8,9,10]. These consequences of delirium led to increased research on delirium, including in the neurosurgical population [5, 7, 9, 11].
In order to assess the current knowledge regarding the diagnostic work-up, incidence, risk factors and health outcomes associated with post-operative delirium in hospitalized neurosurgical patients with primary brain pathologies, we conducted a systematic review and meta-analysis.
Methods
Protocol and registration
This study follows the guideline from Meta-Analysis of Observational Studies in Epidemiology (MOOSE) [12] and is registered in the PROSPERO database (CRD42020166656).
Search strategy
The literature search was conducted with a dedicated biomedical information specialist. The electronic databases Embase, Medline, Web of Science, PsycINFO and Cochrane Central were searched from date of inception through March 31st, 2021 (Appendix 1).
Study selection and eligibility criteria
Two reviewers (PK/EK) independently screened the title/abstract according to a standardized protocol [13]. Of note, we have decided to only include patients that underwent intracranial surgery (with and/or without requirement of bone flap removal) to assess delirium as a post-operative complication to improve the uniformity of the study population, which is a minor adaptation from the original protocol as registered in PROSPERO. Prospective, retrospective cohort studies and randomized controlled trials (RCTs) were included. Exclusion criteria were extra-cranial neurosurgical procedures, case series with a sample size of < 10 patients and English full text not available. Full-text screening required a clear number of patients that underwent intracranial surgery and reproducible diagnosis of delirium, with or without the use of a validated tool (e.g. just mentioning delirium without detail on diagnostic assessment would lead to exclusion).
Data extraction and data items
Data, including author name, year of publication, study design, baseline characteristics, method of delirium assessment, cohort size (including incidence of delirium), risk factors and health outcomes, were extracted independently by the same two reviewers (PK/EK). The primary outcome was method of delirium assessment (validated vs non-validated tools, daily frequency and follow-up). Secondary outcomes included the incidence, risk factors and delirium-related health outcomes associated with post-operative delirium. In case of a RCT, only data of the control group were used. Risk factors and health-related outcomes were evaluated in studies using validated delirium assessment tools (i.e. delirium assessment tools validated within any hospital-based population) [14].
Risk of bias assessment
The same two reviewers (PK/EK) independently evaluated the risk of bias. For RCTs, the Cochrane Collaboration’s risk of bias tool was used [15]. Non-randomized trials were evaluated using the Newcastle–Ottawa Scale (NOS). The NOS’s was adapted, after individually appraising the first five articles, due to its poor inter-observer reliability (Cohen’s kappa = 0.29) (Appendix 2) [16, 17]. The grade of certainty across studies was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Statistical analysis
Descriptive statistics were presented as counts (n, %) and means (standard deviation (SD)). Medians, in case of skewed variables, were used as approximation of the mean. Interquartile ranges (IQR) were divided by 1.35 as approximation of the SD. Reported confidence intervals (95%CI) were used to approximate the SD (= ((CI upper limit–CI lower limit)/3.92)*(root square of the cohort size)). The widths of reported ranges were divided by four as approximation of the SD.
Meta-analysis of proportions was performed using the random effects model with the restricted maximum likelihood method, since within and between-study variance was expected. Proportions were defined as the fraction of patients with delirium. Before pooling, all data were transformed, using the Freeman–Tukey double arcsine transformation, to correct for extreme proportions (e.g. < 0.2 and > 0.8) and small sample sizes [18]. Heterogeneity was assessed using the I2 statistics. Outliers were identified by screening for externally studentized residuals of > 3 and excluded if the outlier caused significant changes in the meta-analysis [19]. Subgroup analysis was performed based on clinical features and delirium diagnosis method. Delirium-associated significant multivariate risk factors and health outcomes were presented as odds ratio’s (ORs) with CIs. Meta-regression was performed for risk factors if ≥ 8 studies were available. We did qualitative analysis for delirium-related risk factors and health outcomes, when studies reported multivariable associations. Data were analysed using R version 4.0.0, and a p value of < 0.05 was considered statistically significant.
Results
Systematic search
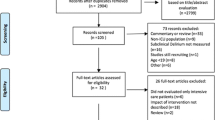
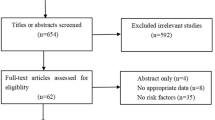
Our search, last update conducted on March 31st, 2021, yielded 6974 studies (Appendix 3). A total of 4290 studies were screened on the title/abstract. Eventually, 47 studies were assessed full text, of which 27 excluded: delirium diagnosis not reproducible (ns (number of studies) = 9), [20,21,22,23,24,25,26,27,28] full text not found (ns = 3), [29,30,31] duplicate (ns = 3), [18, 32] paediatric patients (ns = 1) [33], overlapping populations (ns = 3), [4, 34, 35] no delirium assessment (ns = 1), [36] no original data (ns = 2) [37, 38] and an unclear number of patients undergoing intracranial surgery (ns = 5) [39,40,41,42,43]. Finally, 20 paper were included in the qualitative analysis and 18 papers in the quantitative analysis (np (number of patients) = 5083).
Study and patient characteristics
Table 1 describes the study and patient characteristics. Two RCTs, seven prospective and eleven retrospective cohort studies were included. Disease type for patients undergoing intracranial surgery were categorized in mixed (33.9%, np = 1478), [4, 10, 21, 32, 39, 41,42,43,44,45,46,47,48] functional neurosurgery (26.8%, np = 552), [11, 46, 49,50,51] neurovascular (10.5%, np = 145), [52,53,54] neuro-oncology (18.4%, np = 1969) [5, 7, 55], traumatic brain injury (TBI) (4.3%, np = 27) [56, 57] and microvascular decompression (MVD) (6.2%, np = 912) [9]. The mixed group included neurovascular, neuro-oncologic, TBI or hydrocephalus operations and functional neurosurgery (solely deep brain stimulation (DBS) in patients with Parkinson’s disease). Twelve studies assessed delirium in neurosurgical patients in the nursing ward, [7, 9,10,11, 23, 39, 41,42,43, 45, 46, 49,50,51,52, 56, 57] six studies in the ICU [4, 32, 46,47,48, 53, 54] and two studies in both [44, 55]. Six studies did not specify the number of patients undergoing craniotomy (i.e. requiring removal) [10, 21, 39, 44, 46,47,48, 52, 54, 56, 57]. Six studies did not report age, and seven studies did not report gender within the intracranial operated cohort [45, 47,48,49, 52,53,54]. Pooled age in years (mean/SD, ns = 14) [4, 5, 7, 9,10,11, 32, 44, 46, 49,50,51, 55, 56] and percentage of males (ns = 13) [5, 7, 9,10,11, 32, 44, 46, 50, 51, 55, 56] of the remaining studies were 60.32% (4.47) and 49.6%, respectively.
Delirium diagnosis
Fourteen (70.0%) studies used validated delirium assessment tools (Table 2). One (5.0%) study confirmed delirium, in patients using Delirium Observation Screening Scale (DOS) scores > 2, in combination with the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria [9, 39]. Most studies (ns = 9 (45.0%)) used the Confusion Assessment Method (CAM) or the modified version for the intensive care unit (CAM-ICU) as a diagnostic or screening tool. The CAM(-ICU) in all studies was defined as positive for delirium when three out of four items were scored positive [4, 7, 32, 42, 45,46,47,48, 51, 53, 55]. Two (10.0%) studies assessed delirium using the Intensive Care Delirium Screening Checklist (ICDSC) [10, 39, 41, 54]. One (5.0%) study, assessed delirium using the Neelon and Champagne (NEECHAM) Confusion Scale, defined delirium as positive in case of once a score of < 24 or a score of < 27 for 2 consecutive days [52]. One (5.0%) study used the Nursing Delirium Screening Scale (Nu-DESC), as an alternative for the CAM-ICU, and considered delirium positive in case of a score ≥ 2 [44].
Six (30.0%) studies used non-validated, but reproducible, screening tools for delirium. One study, assessing delirium with either the Mini-Mental State Examination (MMSE) or CAM-ICU, did not separately report values for the CAM-ICU and was therefore considered non-validated [45]. The remaining studies predefined their tools based on own defined criteria [11, 21, 49, 50, 56].
A follow-up period for delirium assessment was reported in all but one study [56], which varied from 24 h to 30 days. Frequency of daily delirium screening was specified in eight (40.0%) studies: three times per day (ns = 2), [32, 39, 43, 55] twice per day (ns = 4) [10, 46, 48, 53] and once per day (ns = 2) [4, 46, 51, 57].
Incidence of delirium
One study did not report the incidence of delirium within the operated population [48]. Meta-analysis was conducted for 18 studies, after excluding one outlying study (Appendix 4), [54] resulting in a pooled incidence of post-operative delirium after intracranial surgery of 19.0% (np = 5083; 0.19; CI 0.12–0.26) (Figs. 1 and 2) [5, 7, 9,10,11, 32, 44,45,46,47, 49,50,51,52,53, 55, 56]. The mean/SD of onset in days, reported in three studies, was 2.8/0.6 [9, 45, 53]. Four studies, distinguishing the delirium subtypes, reported the hypoactive form in 38.9–68.1%, hyperactive form in 17.2–50.8% and the mixed form in 7.57–29.6% of the patients [4, 5, 53, 55].
Subgroup analysis
Delirium assessment tools
The incidence of delirium in studies using validated tools and non-validated tools was 20.0% (np = 4269; 0.20; CI 0.14–0.27) [7, 9, 10, 32, 44, 46, 47, 51, 52, 55] and 17.0%, respectively (np = 814; 0.17; CI 0.07–0.30) (Fig. 3) [5, 11, 45, 49, 50, 56, 57]. The delirium incidence rates were 19.0%, 15.0%, 24.0% and 30.0% when using the CAM(-ICU), [4, 7, 32, 44, 46,47,48, 51, 53, 55] ICDSC, [10, 39] DOS [9, 43] and NEECHAM, [52] respectively.
Frequency and follow-up of daily delirium assessment
Pooled analysis of studies which did not report frequency of delirium assessment resulted in an incidence of 18.0% (N = 2746; 0.18; CI 0.11–0.25), [5, 7, 9, 11, 23, 44,45,46, 49, 50, 52, 56] 20.0% (np = 1029; 0.20; CI 0.17–0.22) [46, 47, 51, 57] in case of screening once per day, 36.0% (N = 350; 0.36; CI 0.17–0.57) [10, 46,47,48, 53] in case of screening twice per day and 5.0% (np = 958; 0.05; CI 0.00–0.28) [8, 32] in case of screening three times per day. Pooled analysis of studies assessing delirium during < 3 days resulted in an incidence of 18.0% (np = 3775; 0.18; CI 0.12–0.24) [5, 7, 9, 11, 32, 44,45,46, 49,50,51,52, 56, 57] and in 21.0% (np = 1308; 0.21; CI 0.07–0.40) in case of ≥ 3 days [4, 5, 7, 9,10,11, 32, 39, 43,44,45, 47,48,49,50, 52, 53, 55].
Clinical features
The pooled analysis of patients undergoing craniotomy (i.e. requiring bone flap removal) led to a delirium incidence of 15.0% (np = 2954; 0.15; CI 0.04–0.32) [7, 9, 23, 32, 52, 53, 55]. The incidence of delirium varied per type of neurosurgical disease; the incidence of 8.0% in neuro-oncologic patients (np = 1969; 0.08; CI 0.03–0.15), [7, 23, 55] 20% in functional neurosurgical patients (np = 552; 0.20; 0.12–0.30), 24.0% in microvascular decompression patients (np = 912; 0.24; CI 0.22–0.27), [9] 19.0% in TBI patients (np = 27;0.19; CI 0.06–0.36), [56, 57] 42.0% in neurovascular patients (np = 145; 0.42; CI 0.18–0.67) [52, 53] and 17.0% in the mixed neurosurgical population (np = 1478; 0.17; CI 0.09–0.28) [4, 10, 32, 39, 43,44,45, 47, 48]. Delirium incidence in patients admitted to the ICU, ward or both were respectively 24.0% (np = 1150; 0.24; CI 0.08–0.46), [4, 5, 7, 9,10,11, 32, 39, 43, 45, 47, 49, 50, 52, 53, 56, 57], 17.0% (np = 2805; 0.17; 0.11–0.25) [7, 9,10,11, 23, 45, 46, 49,50,51,52, 56] and 18.0% (np = 1128; 0.19;0.11–0.26) [4, 32, 44, 47, 48, 53, 55].
Risk factors and health outcome
Risk factors
Independent risk factors from eight studies are presented in Table 3. Age was reported as significant risk factor in four, [7, 44, 46, 55] male gender in three [9, 51, 55] and sleep disturbances [9, 46] and longer surgery duration in two studies [4, 55]. All other risk factors were each described in only one study.
Meta-regression
Meta-regression was performed for age and gender (from baseline characteristics), for which no significant correlation was found with delirium occurrence (p = 0.91, respectively p = 0.37) (Figs. 4 and 5) [4, 7, 9, 10, 32, 43, 44, 46,47,48, 51,52,53, 55].
Health outcomes
Health outcomes were assessed in four studies. Table 4 illustrates health outcomes related to delirium. Delirium was significantly associated with restraint/fixation of patients in three studies [10, 53, 55, 58] and with an unfavourable Glasgow Outcome Scale at discharge [7], increased length of ICU, catheterization and disease in one study [55].
Risk of bias
An overview of the risk of bias assessment is presented in Appendix 5. The quality of evidence was considered poor to moderate. The risk of bias in the study of Greenberg et al. [32] was considered with ‘some concerns’ due to unclear allocation concealment and missing data. The risk of bias in the study of Mokhtari et al. [48] was considered high due to incomplete data and exclusion of patients admitted to the ICU after randomization.
The quality of evidence in the cohort studies was poor in 11 (55.0%) studies, fair in three (15.0%) studies and good in six (30.0%) studies. Only three studies assessed delirium at baseline [21, 52, 55, 56]. Inter-observer reliability between the two researchers (PK/EK) for the NOS was ‘moderate’ (Cohen’s kappa (range); 0.62 (0.50–0.73)) 16].
GRADE certainty rating
The quality of evidence was moderate for the studies included in the meta-analysis. Imprecision was considered moderate since the 95%CI was wide. Inconsistency was considered high since the 95%CI of the individual studies in the meta-analysis did not all overlap, which is confirmed by the heterogeneity test (I2 = 95.0%, p < 0.01). The risk for indirectness was considered moderate; although the type of neurosurgical patients included (neuro-oncology, neurovascular etc.) did differ, delirium was investigated in the population of interest. The risk for publication bias is considered high illustrated by the asymmetrical scattering in the funnel plot (Fig. 2). Based on the previous, the GRADE certainty rating is low to moderate.
Discussion
To our knowledge this is the first systematic review and meta-analysis studying delirium in patients undergoing intracranial surgery. We found an overall incidence of 19%, but the diagnostic method to assess the presence of delirium and the type of neurosurgical patients were highly variable. Although the incidence rate is significant, the current evidence is too limited to draw firm conclusions on risk factors and health outcomes associated with delirium in this specific group of patients.
In this review, it was not possible to investigate which delirium assessment tool was most suitable for the neurosurgical population, since diagnostic accuracy was not determined in any of the included studies and no specific reference standard exists for this population, apart from the DSM criteria. The CAM was mostly used as a screening tool, which is considered a reliable assessment instrument for delirium in postsurgical patients [59]. The second most used assessment tool in this review was the ICDSC, a tool primarily developed for the ICU [60]. The CAM-ICU has a higher sensitivity and specificity compared to the ICDSC (80% and 96%, respectively, 74% and 82%) in critically ill patients [61], which might explain the slightly higher incidence (CAM-ICU; 19%, ICDSC; 15%). Future studies should further validate these screening tools as certain symptoms specific to the neurosurgical patient overlap with diagnostic criteria of delirium.
A considerable proportion of the studies in our review used non-validated tools [5, 11, 21, 45, 49, 50, 56, 57]. Most of these studies were retrospective with delirium assessment based on ‘positive’ symptoms [11]. These assessments might fail to recognizing delirium, especially the hypoactive type which compromises 26–58% of delirium in this population [4, 5, 23, 53]. Structured screening done once vs twice per day increased the incidence (20.0 vs 36.0%), but in studies screening three times per day, the incidence surprisingly decreased (5.0%). This might have been caused by one study, reporting 0% incidence with short follow-up time (within 24 h) [32]. Still, future studies should assess delirium at several moments per day, as delirium fluctuates and infrequent assessments might falsely decrease delirium detection [1].
In our study, post-operative delirium after intracranial surgery occurred in 19% (range 5%–37%), comparable to the pooled incidence (12–43%) reported by Patel et al., evaluating delirium in neurocritical care patients [62]. The difference in incidence between the ICU compared to the ward was not as large as we expected (24.0 vs 17.0%). Explanations for this might include all ICU patients were diagnosed with a valid delirium assessment tool, as opposed to only half of the patients on the ward, and use of sedatives might artificially decrease the incidence of delirium since in drug-induced coma, delirium is by definition undetectable. The clear criteria of validated delirium screening tools compared to the more loose non-validated criteria in many other studies might have affected these incidence rates.
The highest incidence of delirium was found in patients undergoing neurovascular surgery (42%) [52, 53]. A possible explanation for this may be cerebral ischaemia, hypoxia and oxidative stress, induced by, e.g. temporary clipping and bypass techniques, which are described as mechanisms in the pathophysiology of delirium [2]. Moreover, neurovascular procedures are often characterized by a relative long duration of anaesthesia and require frequent post-operative sedation and mechanical ventilation [4, 43]. A relatively lower incidence was observed in the TBI study, possibly caused by the low surgical invasiveness in this cohort, as only patients undergoing burr hole drainage without craniotomy (i.e. requiring boneflap removal) were included [56].
We did not find a correlation between age and delirium, in contrary to literature in other populations [63]. An explanation might be the relatively low range in age (47.8–64.1 years) of the patients in the studies, which is representative of the neurosurgical population. Moreover, the meta-regression analysis might have been underpowered due to high heterogeneity [64]. On the other hand, age might be a less relevant factor after intracranial surgery as it was only described as a risk factor for delirium in four studies [7, 44, 46, 55] and not confirmed in the other five studies [9, 10, 38, 46].
Limitations
The most important limitation in our study is the high heterogeneity of our included studies caused by the differences in delirium assessment methods and clinical differences. Moreover, scattering in the funnel plot indicates a high probability of publication bias. Hence, the findings, especially the quantitative analysis, of this review should be interpreted carefully and be regarded as hypothesis-generating.
Future research
Future research should assess delirium at several moments per day and focus on the validation of structural delirium assessment tools and the prognostic relevance of delirium for clinical outcomes and surgical complications in neurosurgical patients. This is desirable before interventional trials are undertaken to assess optimal management. Furthermore, our analyses indicate that the definition of delirium after intracranial surgery requires consensus to enhance further research. Further, details on depth and length of anaesthesia for surgical procedures and timing of delirium assessments relative to the surgery should be taken into account, to distinguish anaesthesia effects from the impact of structural cerebral pathologies on the phenomenology of delirium.
Conclusion
This is the first systematic review and meta-analysis on delirium after intracranial surgery in neurosurgical patients. Delirium is a frequently occurring adverse event in the neurosurgical clinical practice, but limited consensus exists on the diagnostic criteria. Future research should focus on validating delirium assessment methods in the neurosurgical population and define the prognostic impact of delirium.
Data availability
Data is available and may be requested.
Code availability
Not applicable.
References
American Psychiatric A. American Psychiatric Association DSM-5 Task Force (2013): diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association;
Maldonado JR (2013) Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry 21(12):1190–1222. https://doi.org/10.1016/j.jagp.2013.09.005
Stienen MN (2019) Delirium in neurosurgery. Acta Neurochir (Wien). https://doi.org/10.1007/s00701-019-03928
Wang CM, Huang HW, Wang YM, He X, Sun XM, Zhou YM et al (2020) Incidence and risk factors of postoperative delirium in patients admitted to the ICU after elective intracranial surgery: a prospective cohort study. Eur J Anaesthesiol. https://doi.org/10.1097/EJA.0000000000001074
Flanigan PM, Jahangiri A, Weinstein D, Dayani F, Chandra A, Kanungo I et al (2018) Postoperative delirium in glioblastoma patients: risk factors and prognostic implications. Neurosurgery 83(6):1161–1172. https://doi.org/10.1093/neuros/nyx606
Hosker C, Ward D (2017) Hypoactive delirium. BMJ 357:j2047. https://doi.org/10.1136/bmj.j2047
Budėnas A, Tamašauskas Š, Šliaužys A, Navickaitė I, Sidaraitė M, Pranckevičienė A et al (2018) Incidence and clinical significance of postoperative delirium after brain tumor surgery. Acta Neurochir 160(12):2327–2337. https://doi.org/10.1007/s00701-018-3718-2
Chen L, Xu M, Li GY, Cai WX, Zhou JX (2014) Incidence, risk factors and consequences of emergence agitation in adult patients after elective craniotomy for brain tumor: a prospective cohort study. PLoS ONE 9(12):e114239. https://doi.org/10.1371/journal.pone.0114239
He Z, Cheng H, Wu H, Sun G, Yuan J (2019) Risk factors for postoperative delirium in patients undergoing microvascular decompression. PLoS ONE 14(4):e0215374. https://doi.org/10.1371/journal.pone.0215374
Matano F, Mizunari T, Yamada K, Kobayashi S, Murai Y, Morita A (2017) Environmental and clinical risk factors for delirium in a neurosurgical center: a prospective study. World Neurosurg 103:424–430. https://doi.org/10.1016/j.wneu.2017.03.139
Tanaka M, Tani N, Maruo T, Oshino S, Hosomi K, Saitoh Y et al (2018) Risk factors for postoperative delirium after deep brain stimulation surgery for parkinson disease. World neurosurgery 114:e518–e523. https://doi.org/10.1016/j.wneu.2018.03.021
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–12. https://doi.org/10.1001/jama.283.15.2008
Bramer WMRM, Mast F (2018) Evaluation of a new method for librarian-mediated literature searches for systematic reviews. Res Synth Methods 9(4):510–520. https://doi.org/10.1002/jrsm.1279
De Jayita, Wand A (2015) Delirium screening: a systematic review of delirium screening tools in hospitalized patients. Gerontologist 55(6):1079–99. https://doi.org/10.1093/geront/gnv100
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Hartling L, Hamm M, Milne A, Vandermeer B, Santaguida PL, Ansari M, et al. Validity and inter-rater reliability testing of quality assessment instruments. 2012/04/27 ed2012 Mar.
Wan N. How to conduct a meta-analysis of porportions in R: a comprehensive tutorial. 2018.https://doi.org/10.13140/RG.2.2.27199.00161
B.G. Tabachnick LSF. Using multivariate statistics: Pearson; 2013.
Bouajram RH, Bhatt K, Croci R, Baumgartner L, Puntillo K, Ramsay J et al (2019) Incidence of dexmedetomidine withdrawal in adult critically ill patients: a pilot study. Crit Care Explor 1(8):e0035. https://doi.org/10.1097/CCE.0000000000000035
Brown EG, Josephson SA, Anderson N, Reid M, Lee M, Douglas VC (2018) Evaluation of a multicomponent pathway to address inpatient delirium on a neurosciences ward. BMC Health Serv Res 18(1):106. https://doi.org/10.1186/s12913-018-2906-3
Cao J, Jiao J, Du Z, Xu W, Sun B, Li F et al (2017) Combined hyperactive dysfunction syndrome of the cranial nerves: a retrospective systematic study of clinical characteristics in 44 patients. World Neurosurg 104:390–7 (S1878-8750(17)30708-8)
Flanigan PM, Jahangiri A, Kuang R, Truong A, Choi S, Chou A et al (2018) Developing an algorithm for optimizing care of elderly patients with glioblastoma. Neurosurgery 82(1):64–75. https://doi.org/10.1093/neuros/nyx148
Moeini M, Mahmoudian SN, Khalifezadeh A, Pour AH (2010) Reviewing time intervals from onset of the symptoms to thrombolytic therapy in patients with ST segment elevation myocardial infarction (STEMI). Iran J Nurs Midwifery Res 15(Suppl 1):379–385
Lukasiewicz AM, Grant RA, Basques BA, Webb ML, Samuel AM, Grauer JN (2016) Patient factors associated with 30-day morbidity, mortality, and length of stay after surgery for subdural hematoma: a study of the American College of Surgeons National Surgical Quality Improvement Program. J Neurosurg 124(3):760–766. https://doi.org/10.3171/2015.2.JNS142721
Pledl HW, Hoyer C, Rausch J, Ebert AD, Seiz M, Arp M et al (2016) Decompressive hemicraniectomy in malignant middle cerebral artery infarction: the “real world” beyond studies. Eur Neurol 76(1–2):48–56. https://doi.org/10.1159/000446564
Shinozaki G, Braun PR, Hing BWQ, Ratanatharathorn A, Klisares MJ, Duncan GN et al (2018) Epigenetics of delirium and aging: potential role of DNA methylation change on cytokine genes in glia and blood along with aging. Front Aging Neurosci 10:311. https://doi.org/10.3389/fnagi.2018.00311
Starmark JE, Stalhammar D, Holmgren E, Rosander B (1988) A comparison of the Glasgow Coma Scale and the Reaction Level Scale (RLS85). J Neurosurg 69(5):699–706. https://doi.org/10.3171/jns.1988.69.5.0699
Kim HWKH (2015) The effectiveness of combination therapy with quetapine and valproic acid in delirium with aggressive tendency: a prospective, controlled trial study. Eur Neuropsychopharmacol 25:S591–S592
Mahant N EE, Owler B. . Outcomes of shunting for idiopathic normal pressure hydrocephalus (iNPH) Movement Disorders 2013;;28:S279-.https://doi.org/10.1227/01.neu.0000168187.01077.2f
Mahta A MA, Reznik MM, Park SS, Agarwal SS, Roh DD. Post-operative hemorrhage: a possible predictor of delirium in brain tumor patients. Neurocritical Care. 2016
Greenberg S, Murphy GS, Avram MJ, Shear T, Benson J, Parikh KN et al (2017) Postoperative intravenous acetaminophen for craniotomy patients: a randomized controlled trial. World neurosurgery. https://doi.org/10.1016/j.wneu.2017.10.021
George Brendan P, Vakkalanka J Priyanka, Harland Karisa K, Faine Brett, Rewitzer Stacey, Zepeski Anne, Fuller Brian M, Mohr Nicholas M, Ahmed Azeemuddin (2019) Sedation depth is associated with increased hospital length of stay in mechanically ventilated air medical transport patients: a cohort study. J Prehospi Emerg Care 24(6):783–92. https://doi.org/10.1080/10903127.2019.1705948
Boettger S, Zipser CM, Bode L, Spiller T, Deuel J, Osterhoff G, et al. The prevalence rates and adversities of delirium: too common and disadvantageous. Palliat Support Care. 2020:1–9.https://doi.org/10.1017/s1478951520000632
Schubert M, Schürch R, Boettger S, Nuñez DG, Schwarz U, Bettex D, Jenewein J, Bogdanovic J, Staehli ML, Spirig R, Rudiger A (2018) A hospital-wide evaluation of delirium prevalence and outcomes in acute care patients - a cohort study. BMC Health Serv Res. https://doi.org/10.1186/s12913-018-3345-x
Herrero S, Carrero E, Valero R, Rios J, Fabregas N (2017) Postoperative surveillance in neurosurgical patients - usefulness of neurological assessment scores and bispectral index. Rev Bras Anestesiol 67(2):153–165. https://doi.org/10.1016/j.bjan.2016.12.001
Gelinas C, Puntillo KA, Levin P, Azoulay E (2017) The behavior pain assessment tool for critically ill adults: a validation study in 28 countries. Pain 158(5):811–821
He X, Cheng KM, Zhang L, Gu H, Qu X, Xu Y et al (2020) Dexmedetomidine for the prevention of postoperative delirium in patients after intracranial operation for brain tumours (DEPOD study): a study protocol and statistical plan for a multicentre randomised controlled trial. BMJ Open 10(11):e040939. https://doi.org/10.1136/bmjopen-2020-040939
Çlnar F, Eti AF (2019) Evaluation of postoperative delirium: validity and reliability of the nursing delirium screening scale in the Turkish language. Dement Geriatr Cogn Dis Extra 9(3):362–373. https://doi.org/10.1159/000501903
Patel S, Carey C, Cotter D, Murphy KC (2020) A psychoeducational intervention increases use of a delirium protocol by neurologists and neurosurgeons in patients with brain disorders. Ir J Psychol Med. https://doi.org/10.1017/ipm.2020.98
Yu A, Teitelbaum J, Scott J, Gesin G, Russell B, Huynh T et al (2013) Evaluating pain, sedation, and delirium in the neurologically critically ill-feasibility and reliability of standardized tools: a multi-institutional study. Crit Care Med 41(8):2002–2007. https://doi.org/10.1097/CCM.0b013e31828e96c0
Zipprich HM, Arends MC, Schumacher U, Bahr V, Scherag A, Kwetkat A et al (2020) Outcome of older patients with acute neuropsychological symptoms not fulfilling criteria of delirium. J Am Geriatr Soc. https://doi.org/10.1111/jgs.16422
Zipser Z, Deuel J, Ernst J, Schubert M, von Kanel R, Bottger S (2019) The predisposing and precipitating risk factors for delirium in neurosurgery: a prospective cohort study of 949 patients. Acta Neurochir (Wien) 161(7):1307–1315. https://doi.org/10.1007/s00701-019-03927-z
Morshed RA, Young JS, Safaee M, Sankaran S, Berger MS, McDermott MW et al (2019) Delirium risk factors and associated outcomes in a neurosurgical cohort: a case-control study. World Neurosurg 126:930–936. https://doi.org/10.1016/j.wneu.2019.03.012
Oh YS, Kim DW, Chun HJ, Yi HJ (2008) Incidence and risk factors of acute postoperative delirium in geriatric neurosurgical patients. J Korean Neurosurg Soc 43(3):143–148. https://doi.org/10.3340/jkns.2008.43.3.143
Wang XQ, Zhuang HX, Zhang LX, Chen X, Niu CS, Zhao M (2019) Nomogram for predicting postoperative delirium after deep brain stimulation surgery for Parkinson’s disease. World Neurosurg 130:e551–e557. https://doi.org/10.1016/j.wneu.2019.06.151
Wang J, Ji Y, Wang N, Chen W, Bao Y, Qin Q, et al. Establishment and validation of a delirium prediction model for neurosurgery patients in intensive care. Int J Nurs Pract. 2020:e12818.https://doi.org/10.1111/ijn.12818
Mokhtari M, Farasatinasab M, Jafarpour Machian M, Yaseri M, Ghorbani M, Ramak Hashemi SM et al (2020) Aripiprazole for prevention of delirium in the neurosurgical intensive care unit: a double-blind, randomized, placebo-controlled study. Eur J Clin Pharmacol. https://doi.org/10.1007/s00228-019-02802-1
Carlson JD, Neumiller JJ, Swain LDW, Mark J, McLeod P, Hirschauer J (2014) Postoperative delirium in Parkinson’s disease patients following deep brain stimulation surgery. J Clin Neurosci 21(7):1192–1195. https://doi.org/10.1016/j.jocn.2013.12.007
Lange M, Zech N, Seemann M, Janzen A, Halbing D, Zeman F et al (2015) Anesthesiologic regimen and intraoperative delirium in deep brain stimulation surgery for Parkinson’s disease. J Neurol Sci 355(1–2):168–173. https://doi.org/10.1016/j.jns.2015.06.012
Zhan L, Wang XQ, Zhang LX (2020) Nomogram model for predicting risk of postoperative delirium after deep brain stimulation surgery in patients older than 50 years with Parkinson disease. World Neurosurg 139:e127–e135. https://doi.org/10.1016/j.wneu.2020.03.160
Harasawa N, Mizuno T (2014) A novel scale predicting postoperative delirium (POD) in patients undergoing cerebrovascular surgery. Arch Gerontol Geriatr 59(2):264–271. https://doi.org/10.1016/j.archger.2014.05.007
Wang J, Ji Y, Wang N, Chen W, Bao Y, Qin Q et al (2017) Risk factors for the incidence of delirium in cerebrovascular patients in a neurosurgery intensive care unit: a prospective study. J Clin Nurs 27(1–2):407–415. https://doi.org/10.1111/jocn.13943
Hosoya R, Sato Y, Ishida E, Shibamoto H, Hino S, Yokote H et al (2018) Association between delirium and prehospitalization medication in poststroke patients. J Stroke Cerebrovasc Dis 27(7):1914–1920. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.02.038
Chen H, Jiang H, Chen B, Fan L, Shi W, Jin Y et al (2020) The incidence and predictors of postoperative delirium after brain tumor resection in adults: a cross-sectional survey. World Neurosurg 140:e129–e139. https://doi.org/10.1016/j.wneu.2020.04.195
Ogasawara K, Ogawa A, Okuguchi T, Kobayashi M, Suzuki M, Yoshimoto T et al (2000) Postoperative hyperperfusion syndrome in elderly patients with chronic subdural hematoma. Surg Neurol 54(2):155–159. https://doi.org/10.1016/s0090-3019(00)00281-0
Maneewong J, Maneeton B, Maneeton N, Vaniyapong T, Traisathit P, Sricharoen N et al (2017) Delirium after a traumatic brain injury: predictors and symptom patterns. Neuropsychiatr Dis Treat 13:459–465. https://doi.org/10.2147/NDT.S128138
Wang CG, Qin YF, Wan X, Song LC, Li ZJ, Li H (2018) Incidence and risk factors of postoperative delirium in the elderly patients with hip fracture. J Orthop Surg Res 13(1):186. https://doi.org/10.1186/s13018-018-0897-8
Maybrier HR, Mickle AM, Escallier KE, Lin N, Schmitt EM, Upadhyayula RT et al (2018) Reliability and accuracy of delirium assessments among investigators at multiple international centres. BMJ Open 8(11):e023137. https://doi.org/10.1136/bmjopen-2018-023137
Boettger S, Garcia Nunez D, Meyer R, Richter A, Rudiger A, Schubert M et al (2018) Screening for delirium with the Intensive Care Delirium Screening Checklist (ICDSC): a re-evaluation of the threshold for delirium. Swiss Med Wkly 148:w14597. https://doi.org/10.4414/smw.2018.14597
Gusmao-Flores D, Salluh JI, Chalhub RA, Quarantini LC (2012) The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care 16(4):R115. https://doi.org/10.1186/cc11407
Patel MB, Bednarik J, Lee P, Shehabi Y, Salluh JI, Slooter AJ et al (2018) Delirium monitoring in neurocritically ill patients: a systematic review. Crit Care Med 46(11):1832–1841. https://doi.org/10.1097/CCM.0000000000003349
Vasilevskis EE, Han JH, Hughes CG, Ely EW (2012) Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol 26(3):277–287. https://doi.org/10.1016/j.bpa.2012.07.003
Reeves BDJ, Higgins JP (2008) Cochrane handbook for systematic reviews of interventions. John Wiley & Sons, Chichester, U.K
Acknowledgements
We would like to thank Dr. W. Bramer for his assistance with the literature search and Dr. D. Rizopoulos for advice on the statistical analysis.
Funding
This study was funded by the Erasmus University Medical Center. The funders of the study had no role in the study design, data collection, data analysis, manuscript preparation and publication decision.
Author information
Authors and Affiliations
Contributions
PK and EK coordinated the systematic review. PK and EK screened the abstracts and full texts. PK and EK wrote the first draft of the manuscript and judged risk of bias in the studies. PK, EK, CD, MJ, MK and AV interpreted the data. PK, EK, CD, MJ, MK, RO and AV critically revised the manuscript. PK, EK, CD, MJ, MK, RO and AV had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval for this study was not required because no animals or patients were involved as this regards a systematic review of literature.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
P. R. Kappen and E. Kakar are contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kappen, P.R., Kakar, E., Dirven, C.M.F. et al. Delirium in neurosurgery: a systematic review and meta-analysis. Neurosurg Rev 45, 329–341 (2022). https://doi.org/10.1007/s10143-021-01619-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-021-01619-w