Abstract
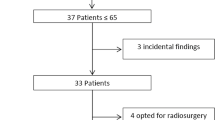
In 10–15% of cases of vestibular schwannoma (VS), age at diagnosis is 40 years or less. Little is known about the differences in natural history, surgical findings, and postoperative outcomes of such younger patients as compared to those of greater age. To analyze clinical and surgical and imaging data of a consecutive series of n = 50 patients with unilateral sporadic VS, aged 40 years or younger — separated in a very young group (15–30 years) and a moderately young group (31–40 years). Retrospective case series. Fifty consecutive patients under 40 years of age underwent microsurgical resection of unilateral sporadic VS via the retrosigmoid approach. The study cohort was subdivided into two groups according to the age range: group A, age range 15–30 years (n = 23 patients), and group B, age range 31–40 years (n = 27 patients). The adherence of VS capsule to surrounding nervous structures and the tendency of the tumors to bleed were evaluated by reviewing video records; the course of the FN in relation to the tumor’s surface was assessed in each case. Microsurgical removal of tumor was classified as total (T), near total (residual tumor volume < 5%), subtotal (residual tumor volume 5–10%), or partial (residual tumor volume > 10%). Mean tumor size of entire cohort was 2.53 (range: 0.6–5.8) cm: 2.84 cm in group A and 2.36 cm in group B (p = NS). Facial nerve course and position within the cerebellopontine angle did not differ significantly between the two groups. At 6-month follow-up, FN functional outcome was HBI-II in 69.5% in group A, versus 96.3% in group B (p < .001). Hearing preservation was achieved in 60.0% of patients of group A and in 58.3% of group B (p = NS). Total and near-total resection was feasible in 95.6% of cases of group A and in 88.9% of group B (p = NS). Tumor capsule was tightly adherent to nervous structures in 69.6% patients of group A and in 22.2% of group B (p < .05). Significant bleeding was encountered in 56.5% of group A tumors, and in 29.6% of group B tumors (p < .01). Microsurgery of VS in patients aged 40 or less is associated with good functional results, and with high rates of total and near total tumor removal. Patients < 30 years of age have more adherent tumor capsules. Furthermore, their tumors exhibit a tendency to larger sizes, to hypervascularization, to profuse intraoperative bleeding and they present worse long-term functional FN results when compared to patients in their fourth decade of life. Our limited experience seems to suggest that a near total resection in very young VS patients with large tumors should be preferred in adherent and hypervascularized cases, in order to maximize resection and preserve function.


Similar content being viewed by others
Availability of data and material
Data available from the first author on demand.
Code availability
None (not applicable).
References
Carlson ML, Smadbeck JB, Link MJ, Klee EW, Vasmatzis G, Schimmenti LA (2018) Next generation sequencing of sporadic vestibular schwannoma. Otol Neurotol 39(9):e860–e871
Caye-Thomasen P, Baandrup L, Jacobsen GK, Thomsen J, Stangerup SE (2003) Immunohistochemical demonstration of vascular endothelial growth factor in vestibular schwannomas correlates to tumor growth rate. Laryngoscope 113(12):2129–2134
Chiluwal AK, Rothman A, Svrakic M, Dehdashti AR (2018) Surgical outcome in smaller symptomatic vestibular schwannomas. Is there a role for surgery? Acta Neurochir (Wien) 160(11):2263–2275
Committee on Hearing and Equilibrium Guidelines for the Evaluation of Hearing Preservation in Acoustic Neuroma (Vestibular Schwannoma): Committee on Hearing and Equilibrium (1995). Otolaryngol Head Neck Surg 113(3):179–180
Di Ieva A, Lee JM, Cusimano MD (2016) Handbook of skull base surgery. Thieme, New York
Eser Ocak P, Dogan I, Ocak U, Dinc C, Baskaya MK (2018) Facial nerve outcome and extent of resection in cystic versus solid vestibular schwannomas in radiosurgery era. Neurosurg Focus 44(3):E3
Ferrara N, Gerber H-P, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9(6):669–676
Gal TJ, Shinn J, Huang B (2010) Current epidemiology and management trends in acoustic neuroma. Otolaryngol Head Neck Surg 142(5):677–681
Goldbrunner R, Weller M, Regis J, Lund-Johansen M, Stavrinou P, Reuss D, Evans DG, Lefranc F, Sallabanda K, Falini A, Axon P, Sterkers O, Fariselli L, Wick W, Tonn JC (2019) EANO guide-line on the diagnosis and treatment of vestibular schwannoma. Neuro Oncol. https://doi.org/10.1093/neuonc/noz153
Goncalves VM, Suhm EM, Ries V, Skardelly M, Tabatabai G, Tatagiba M, Schittenhelm J, Behling F (2021) Macrophage and lymphocyte infiltration is associated with volumetric tumor size but not volumetric growth in the Tübingen schwannoma cohort. Cancers 13:466
Hoshide R, Faulkner H, Teo M, Teo C (2018) Keyhole retrosigmoid approach for large vestibular schwannomas: strategies to improve outcomes. Neurosurg Focus 44(3):E2
House JW, Brackmann DE (1985) Facial nerve grading system. Otolaryngol Head Neck Surg 93(2):146–147
Iwai Y, Ishibashi K, Watanabe Y, Uemura G, Yamanaka K (2015) Functional preservation after planned partial resection followed by Gamma Knife radiosurgery for large vestibular schwannomas. World Neurosurg 84(2):292–300
Kanzaki J, Tos M, Sanna M, Moffat DA (2003) New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol 24(4):642–649
Kohno M, Sato H, Sora S, Miwa H, Yokoyama M (2011) Is an acoustic neuroma an epiarachnoid or subarachnoid tumor? Neurosurgery 68(4):1006–1017
Koos WT, Day JD, Matula C, Levy DI (1988) Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg 88(3):506–512
Mastronardi L, Cacciotti G, Roperto R, Di Scipio E, Tonelli MP, Carpineta E (2016) Position and course of facial nerve and postoperative facial nerve results in vestibular schwannoma microsurgery. World Neurosurg 94:174–180
Mastronardi L, Fukushima T, Campione A (2019) Advances in vestibular schwannoma microneurosurgery, 1st edn. Springer International Publishing, Cham
Mastronardi L, Gazzeri R, Barbieri FR, Roperto R, Cacciotti G, Sufianov A (2020) Postoperative functional preservation of facial nerve in cystic vestibular schwannoma. World Neurosurg. https://doi.org/10.1016/j.wneu.2020.04.018
Matthies C, Samii M, Krebs S (1997) Management of vestibular schwannomas (acoustic neuromas): radiological features in 202 cases-their value for diagnosis and their predictive importance. Neurosurgery 40(3):469–482
Møller MN, Werther K, Nalla A, Stangerup SE, Thomsen J, Bøg-Hansen TC, Nielsen HJ, Cayé-Thomasen P (2010) Angiogenesis in vestibular schwannomas: expression of extracellular matrix factors MMP-2, MMP-9, and TIMP-1. Laryngoscope 120(4):657–662
Nutik SL (1994) Facial nerve outcome after acoustic neuroma surgery. Surg Neurol 41(1):28–33
Nutik SL, Babb MJ (2001) Determinants of tumor size and growth in vestibular schwannomas. J Neurosurg 94(6):922–926
Ohata K, Tsuyuguchi N, Morino M, Takami T, Goto T, Hakuba A, Hara M (2002) A hypothesis of epiarachnoidal growth of vestibular schwannoma at the cerebello-pontine angle: surgical importance. J Postgrad Med 48(4):253–258
Ojemann RG (2001) Retrosigmoid approach to acoustic neuroma (vestibular schwannoma). Neurosurgery 48(3):553–558
Peris-Celda M, Graffeo CS, Perry A, Karezoudis P, Tombers NM, Carlson ML, Link MJ (2019) Main symptom that led to medical evaluation and diagnosis of vestibular schwannoma and patient-reported tumor size: cross-sectional study in 1,304 patients. J Neurol Surg B Skull Base 80(3):316–322
Plotkin SR, Stemmer-Rachamimov AO, Barker FG 2nd, Halpin C, Padera TP, Tyrrell A, Sorensen AG, Jain RK, di Tomaso E (2009) Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med 361(4):358–367
Quiñones-Hinojosa A, Rincon-Torroella J (2017) Video atlas of neurosurgery : contemporary tumor and skull base surgery, 1st edn. Elsevier, New York
Reznitsky M, Petersen MMBS, West N, Stangerup S-E, Cayé-Thomasen P (2019) Epidemiology of vestibular schwannomas - prospective 40-year data from an unselected national cohort. Clin Epidemiol 11:981–986
Roosli C, Linthicum FH Jr, Cureoglu S, Merchant SN (2012) What is the site of origin of cochleovestibular schwannomas? Audiol Neurootol 17(2):121–125
Sameshima T, Morita A, Tanikawa R, Fukushima T, Friedman AH, Zenga F, Ducati A, Mastronardi L (2013) Evaluation of variation in the course of the facial nerve, nerve adhesion to tumors, and postoperative facial palsy in acoustic neuroma. J Neurol Surg B Skull Base 74(1):39–43
Sameshima T, Mastronardi L, Fukushima T (2007) Fukushima’s microanatomy and dissection of the temporal bone, 2nd edn. AF-Neurovideo Inc., Raleigh
Samii M, Matthies C (1997) Management of 1000 vestibular schwannomas (acoustic neuromas): the facial nerve-preservation and restitution of function. Neurosurgery 40(4):684–695
Samii M, Matthies C (1997) Management of 1000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery 40(1):11–23
Sasaki T, Shono T, Hashiguchi K, Yoshida F, Suzuku SO (2009) Histological considerations of the cleavage plane for preservation of facial and cochlear nerve functions in vestibular schwannoma surgery. J Neurosurg 110(4):648–655
Schneider JR, Chiluwal AK, Arapi O, Kwan K, Dehdashti AR (2020) Near total versus gross total resection of large vestibular schwannoma: facial nerve outcome. Oper Neurosurg 19(4):414–421
Starnoni D, Daniel RT, Tuleasca C, George M, Levivier M, Messerer M (2018) Systematic review and meta-analysis of the technique of subtotal resection and stereotactic radiosurgery for large vestibular schwannomas: a “nerve-centered” approach. Neurosurg Focus 44(3):E4
Starnoni D, Giammattei L, Cossu G, Link MJ, Roche PH, Chacko AG, Ohata K, Samii M, Suri A, Bruneau M, Cornelius JF, Cavallo L, Meling TR, Froelich S, Tatagiba M, Sufianov A, Paraskevopoulos D, Zazpe I, Berhouma M, Jouanneau E, Verheul JB, Tuleasca C, George M, Levivier M, Messerer M, Daniel RT (2020) Surgical management for large vestibular schwannomas: a systematic review, meta-analysis, and consensus statement on behalf of the EANS skull base section. Acta Neurochir 162(11):2595–2617
Sughrue ME, Kaur R, Rutkowski MJ, Kane AJ, Yang I, Pitts LH, Parsa AT (2010) A critical evaluation of vestibular schwannoma surgery for patients younger than 40 years age. Neurosurgery 67:1646–1645
Tos M, Thomsen J, Harmsen A (1988) Results of translabyrinthine removal of 300 acoustic neuromas related to tumour size. Acta Otolaryngol Suppl 452:38–51
Troude L, Boucekine M, Montava M, Lavieille JP, Regis JM, Roche PH (2019) Predictive factors of early postoperative and long-term facial nerve function after large vestibular schwannoma surgery. World Neurosurg 127:e599–e608
Uesaka T, Shono T, Suzuki SO, Nakamizo A, Niiro H, Mizoguchi M, Iwaki T, Sasaki T (2007) Expression of VEGF and its receptor genes in intracranial schwannomas. J Neuro-Oncol 83(3):259–266
Winn HR (2017) Youmans and Winn neurological surgery, 7th edn. Elsevier, Philadelphia
Wu H, Zhang L, Han D, Mao Y, Yang J, Wang Z, Jia W, Zhong P, Jia H (2016) Summary and consensus in 7th International Conference on acoustic neuroma an update for the management of sporadic acoustic neuromas. World J Otorhinolaryngol Head Neck Surge 2(4):234–239
Zumofen DW, Guffi T, Epple C, Westermann B, Krähenbühl AK, Zabka S, Taub E, Bodmer D, Mariani L (2018) Intended near-total removal of Koos Grade IV vestibular schwannomas: reconsidering the treatment paradigm. Neurosurgery 82(2):202–210
Author information
Authors and Affiliations
Contributions
LM: study design, study conception, data analysis, manuscript writing.
AC: data extraction, data analysis.
CGS: data extraction, data analysis, radiological measurement.
EC: data extraction, data analysis, radiological measurement.
GC: data extraction, data analysis, statistical analysis.
RR: data analysis, statistical analysis, critical review of the manuscript.
AAS: critical review of the manuscript, study supervision.
KS: critical review of the manuscript, study supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The study involves human participants: therefore, it has been reviewed and approved by local ethics committee of the Hospital. A written consent for scientific treatment of personal data was obtained from any patient before surgery. No potentially identifiable human images or data are presented in this study. All procedures performed in this study were in accordance with the ethical standards of the internal institutional ethics committee (“Comitato Etico Lazio 1” Members of ASLRoma1: Dr. Marco Tubaro, Dr. Teresa Calamia, Dr. Francesco Meo). A written consent was obtained from any patient included in the study.
Consent to participate and consent for publication
All co-authors express formally their consent to participate to this study and to publish it, contributing in different ways.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mastronardi, L., Campione, A., Cacciotti, G. et al. Microsurgical treatment of symptomatic vestibular schwannomas in patients under 40: different results before and after age of 30. Neurosurg Rev 45, 873–882 (2022). https://doi.org/10.1007/s10143-021-01603-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-021-01603-4