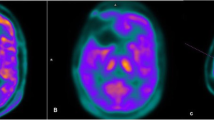
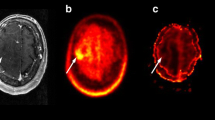
Abstract
The aim of our study was to compare depicted pre-, intra-, and postoperative tumor volume of met-PET, perfusion-weighed MRI (PWI), and Gd-DTPA MRI. Further, to assess their sensitivity and specificity in correlation with histopathological specimen. Inclusion criteria of the prospective study were histological confirmed glioblastoma (GB), age > 18, and eligible for gross total resection (GTR). Met-PET was performed before and after surgery. Gd-DTPA MRI and PWI were performed before, during, and after surgery. A combined 5-aminolevulinic acid (5-ALA) and iMRI-guided surgery was performed. Volumetric analysis was evaluated for all imaging modalities except for 5-ALA. A total of 59 navigated biopsies were taken. Sensitivity and specificity were calculated for Gd-DTPA MRI, PWI, met-PET, and 5-ALA according to the histology of specimen. Met-PET depicted significantly larger tumor volume before surgery (p = 0.01) compared to PWI and Gd-DTPI MRI. We found no significant difference in tumor volume between met-PET and PWI after surgery (p = 0.059). Both PWI and met-PET showed significantly larger tumor volume after surgery when compared to Gd-DTPA (p = 0.018 and p = 0.003, respectively). Intraoperative PWI reading was impaired in 33.3% due to artifacts. Met-PET showed the highest sensitivity for detection of GB with 95%. The lowest sensitivity was found with Gd-DTPA MRI (50%), while 5-ALA and intraoperative PWI showed similar results (69 and 67%). Met-Pet is the imaging modality with the highest sensitivity to detect a residual tumor in GB. Intraoperative PWI seems to have a synergistic effect to Gd-DTPA and 5-ALA. However, its value may be limited by artifacts. Both pre- and intraoperative PWI cannot substitute met-PET in tumor detection.





Similar content being viewed by others
References
Barajas RF, Phillips JJ, Parvataneni R, Molinaro A, Essock-Burns E, Bourne G, Parsa AT, Aghi MK, McDermott MW, Berger MS, Cha S, Chang SM, Nelson SJ (2012) Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR imaging. Neuro-Oncology 14:942–954. https://doi.org/10.1093/neuonc/nos128
Buchmann N, Kläsner B, Gempt J, Bauer JS, Pyka T, Delbridge C, Meyer B, Krause BJ, Ringel F (2016) 18F-Fluoroethyl-l-thyrosine positron emission tomography to delineate tumor residuals after glioblastoma resection: a comparison with standard postoperative magnetic resonance imaging. World Neurosurg 89:420–426. https://doi.org/10.1016/j.wneu.2016.02.032
Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, Raza SM, Pascual-Gallego M, Ibrahim A, Hernandez-Hermann M, Gomez L, Ye X, Weingart JD, Olivi A, Blakeley J, Gallia GL, Lim M, Brem H, Quinoñes-Hinojosa A (2014) Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro-Oncology 16:113–122. https://doi.org/10.1093/neuonc/not137
Coburger J, Engelke J, Scheuerle A, Thal DR, Hlavac M, Wirtz CR, König R (2014) Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA–enhanced intraoperative MRI at the border of contrast-enhancing lesions: a prospective study based on histopathological assessment. Neurosurg Focus 36:E3. https://doi.org/10.3171/2013.11.FOCUS13463
Coburger J, Hagel V, Wirtz CR, König R (2015) Surgery for glioblastoma: impact of the combined use of 5-aminolevulinic acid and intraoperative MRI on extent of resection and survival. PLoS One 10:e0131872. https://doi.org/10.1371/journal.pone.0131872
Coburger J, Scheuerle A, Pala A, Thal D, Wirtz CR, König R (2017) Histopathological insights on imaging results of intraoperative magnetic resonance imaging, 5-aminolevulinic acid, and intraoperative ultrasound in glioblastoma surgery. Neurosurgery 81:165–174. https://doi.org/10.1093/neuros/nyw143
Coburger J, Wirtz CR, König RW (2017) Impact of extent of resection and recurrent surgery on clinical outcome and overall survival in a consecutive series of 170 patients for glioblastoma in intraoperative high field magnetic resonance imaging. J Neurosurg Sci 61:233–244. https://doi.org/10.23736/S0390-5616.16.03284-7
Filss CP, Galldiks N, Stoffels G, Sabel M, Wittsack HJ, Turowski B, Antoch G, Zhang K, Fink GR, Coenen HH, Shah NJ, Herzog H, Langen K-J (2014) Comparison of 18F-FET PET and perfusion-weighted MR imaging: a PET/MR imaging hybrid study in patients with brain tumors. J Nucl Med 55:540–545. https://doi.org/10.2967/jnumed.113.129007
Floeth FW, Sabel M, Ewelt C, Stummer W, Felsberg J, Reifenberger G, Steiger HJ, Stoffels G, Coenen HH, Langen K-J (2010) Comparison of 18F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur J Nucl Med Mol Imaging 38:731–741. https://doi.org/10.1007/s00259-010-1690-z
Galban CJ, Chenevert TL, Meyer CR, Tsien C, Lawrence TS, Hamstra DA, Junck L, Sundgren PC, Johnson TD, Galban S, Sebolt-Leopold JS, Rehemtulla A, Ross BD (2011) Prospective analysis of parametric response map-derived MRI biomarkers: identification of early and distinct glioma response patterns not predicted by standard radiographic assessment. Clin Cancer Res 17:4751–4760. https://doi.org/10.1158/1078-0432.CCR-10-2098
Hatiboglu MA, Weinberg JS, Suki D, Rao G, Prabhu SS, Shah K, Jackson E, Sawaya R (2009) Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery. Neurosurgery 64:1073–1081. https://doi.org/10.1227/01.NEU.0000345647.58219.07
Kato T, Shinoda J, Nakayama N, Miwa K, Okumura A, Yano H, Yoshimura S, Maruyama T, Muragaki Y, Iwama T (2008) Metabolic assessment of gliomas using 11C-methionine, [18F] fluorodeoxyglucose, and 11C-choline positron-emission tomography. AJNR Am J Neuroradiol 29:1176–1182. https://doi.org/10.3174/ajnr.A1008
Kracht LW, Miletic H, Busch S, Jacobs AH, Voges J, Hoevels M, Klein JC, Herholz K, Heiss W-D (2004) Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res 10:7163–7170. https://doi.org/10.1158/1078-0432.CCR-04-0262
Kreth FW, Thon N, Simon M, Westphal M, Schackert G, Nikkhah G, Hentschel B, Reifenberger G, Pietsch T, Weller M, Tonn JC, German Glioma Network (2013) Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol 24:3117–3123. https://doi.org/10.1093/annonc/mdt388
Kubben P, Wesseling P, Lammens M, Schijns OMG, Laak Poort ter M, van Overbeeke J, Santbrink H (2012) Correlation between contrast enhancement on intraoperative magnetic resonance imaging and histopathology in glioblastoma. Surg Neurol Int 3:158. https://doi.org/10.4103/2152-7806.105097
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198. https://doi.org/10.3171/jns.2001.95.2.0190
Law M, Young RJ, Babb JS, Peccerelli N, Chheang S, Gruber ML, Miller DC, Golfinos JG, Zagzag D, Johnson G (2008) Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 247:490–498. https://doi.org/10.1148/radiol.2472070898
Li YM, Suki D, Hess K, Sawaya R (2016) The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg 124:977–988. https://doi.org/10.3171/2015.5.JNS142087
McGirt MJ, Chaichana KL, Attenello FJ, Weingart JD, Than K, Burger PC, Olivi A, Brem H, Quinoñes-Hinojosa A (2008) Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery 63:700–707. https://doi.org/10.1227/01.NEU.0000325729.41085.73 author reply 707–8
McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, Weingart JD, Brem H, Quiñones-Hinojosa AR (2009) Independent association of extent of resection with survival in patients with malignant brain astrocytoma. 110:156–162. https://doi.org/10.3171/2008.4.17536
Pala A, Brand C, Kapapa T, Hlavac M, König R, Schmitz B, Wirtz CR, Coburger J (2016) The value of intraoperative and early postoperative MRI in low-grade glioma surgery a retrospective study. World Neurosurg 93:191–197. https://doi.org/10.1016/j.wneu.2016.04.120
Pichlmeier U, Bink A, Schackert G, Stummer W, ALA-Glioma Study Group (2008) Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro-Oncology 10:1025–1034. https://doi.org/10.1215/15228517-2008-052
Pirotte BJM, Levivier M, Goldman S, Massager N, Wikler D, Dewitte O, Bruneau M, Rorive S, David P, Brotchi J (2009) Positron emission tomography-guided volumetric resection of supratentorial high-grade gliomas: a survival analysis in 66 consecutive patients. Neurosurgery 64:471–481. https://doi.org/10.1227/01.NEU.0000338949.94496.85 discussion 481
Roder C, Bender B, Ritz R, Honegger J, Feigl G, Naegele T, Tatagiba MS, Ernemann U, Bisdas S (2013) Intraoperative visualization of residual tumor: the role of perfusion-weighted imaging in a high-field intraoperative magnetic resonance scanner. Neurosurgery 72:ons151–ons158. https://doi.org/10.1227/NEU.0b013e318277c606 discussion ons158
Roder C, Bisdas S, Ebner FH, Honegger J, Naegele T, Ernemann U, Tatagiba M (2014) Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: high-field iMRI versus conventional and 5-ALA-assisted surgery. Eur J Surg Oncol 40:297–304. https://doi.org/10.1016/j.ejso.2013.11.022
Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 62:753–764. https://doi.org/10.1227/01.neu.0000318159.21731.cf discussion 264–6
Stockhammer F, Misch M, Horn P, Koch A, Fonyuy N, Plotkin M (2009) Association of F18-fluoro-ethyl-tyrosin uptake and 5-aminolevulinic acid-induced fluorescence in gliomas. Acta Neurochir 151:1377–1383. https://doi.org/10.1007/s00701-009-0462-7
Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93:1003–1013. https://doi.org/10.3171/jns.2000.93.6.1003
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen H-J, ALA-Glioma Study Group (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401. https://doi.org/10.1016/S1470-2045(06)70665-9
Tsien C, Galbán CJ, Chenevert TL, Johnson TD, Hamstra DA, Sundgren PC, Junck L, Meyer CR, Rehemtulla A, Lawrence T, Ross BD (2010) Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J Clin Oncol 28:2293–2299. https://doi.org/10.1200/JCO.2009.25.3971
Ulmer S, Liess C, Kesari S, Otto N, Straube T, Jansen O (2008) Use of dynamic susceptibility-contrast MRI (DSC-MRI) to assess perfusion changes in the ipsilateral brain parenchyma from glioblastoma. J Neuro-Oncol 91:213–220. https://doi.org/10.1007/s11060-008-9701-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
An institutional ethics approval was obtained by the local ethical board (Ethikkommission Ulm No: 172/12). The study was conducted according to the international Declaration of Helsinki.
Statement of informed consent
Informed consent was obtained.
Rights and permissions
About this article
Cite this article
Pala, A., Reske, S.N., Eberhardt, N. et al. Diagnostic accuracy of intraoperative perfusion-weighted MRI and 5-aminolevulinic acid in relation to contrast-enhanced intraoperative MRI and 11C-methionine positron emission tomography in resection of glioblastoma: a prospective study. Neurosurg Rev 42, 471–479 (2019). https://doi.org/10.1007/s10143-018-0987-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-018-0987-4