Abstract
Interleukin-1 receptor antagonist (IL-1RA) has been shown to play an important role in cancer progression. However, its pathogenic effects and molecular mechanism in the malignant progression of esophageal squamous cell carcinoma (ESCC) remain largely unknown. This study was designed to explore the function of IL-1RA in ESCC and determine the relationship between IL-1RA and lymph node metastasis in ESCC patients. The clinical relevance of IL-1RA in relation to the clinicopathological features and prognosis of 100 ESCC patients was analyzed. The function and underlying mechanisms of IL-1RA in the growth, invasion, and lymphatic metastasis in ESCC were explored both in vitro and in vivo. The therapeutic effect of anakinra, an IL-1 receptor antagonist, on ESCC was also evaluated in animal experiments. Downregulation of IL-1RA was observed in ESCC tissues and cells and was found to be strongly correlated with pathological stage (P = 0.034) and lymphatic metastasis (P = 0.038). Functional assays demonstrated that upregulation of IL-1RA reduced cell proliferation, migration, and lymphangiogenesis both in vitro and in vivo. Mechanistic studies revealed that overexpression of IL-1RA activated the epithelial-to-mesenchymal transition (EMT) in the ESCC cells through activation of MMP9 and regulation of the expression and secretion of VEGF-C through the PI3K/NF-κB pathway. Anakinra treatment resulted in significant inhibition of tumor growth, lymphangiogenesis, and metastasis. IL-1RA inhibits lymph node metastasis of ESCC by regulating the EMT through activation of matrix metalloproteinase 9(MMP9) and lymphangiogenesis, driven by VEGF-C and the NF-κB signaling pathway. Anakinra may be an effective drug for the inhibition of ESCC tumor formation and lymph node metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer is one of the seven most aggressive tumors and has the sixth highest cancer-associated mortality rate globally (Bray et al. 2018). According to histopathology, esophageal squamous cell carcinoma (ESCC) is the most common form of esophageal carcinoma (EC) (Hao et al. 2016) and occurs predominantly in Asian populations (Gao et al. 2014; Song et al. 2014). Owing to the large population and high incidence of ESCC, the number of cases in China makes up more than half of those worldwide (Shah et al. 2019). Although patients with ESCC are treated according to current standard guidelines, some with locally advanced ESCC have an unsatisfactory overall survival (OS) (Lordick et al. 2016), with the global 5-year OS rate around 15–25% (Rice et al. 2017). Current treatment options for ESCC generally depend on its stage, with endoscopic resection for mucosal lesions versus esophagectomy for submucosal lesions the primary treatment option for early disease. Extensive studies have recorded that neoadjuvant therapy combined surgery is beneficial in improving the prognosis of locally advanced ESCC (Yang et al. 2018a). Over the past years, technology advancements have comprehensively characterized the genomic landscape in ESCC, and targeted therapy has attracted increased attention (Fatehi Hassanabad et al. 2020). The principal reason for the poor prognosis of ESCC is lymph node metastasis (Smyth et al. 2017). Hence, understanding the molecular mechanism underlying ESCC lymph node metastasis and identifying sensitive and specific molecular biomarkers will help its early diagnosis and prognosis. Our study explored novel targets for the diagnosis and treatment of esophageal cancer by exploring the imbalance between IL-1RA and IL-1A, which leads to tumor-induced lymphangiogenesis, with a stronger basis for clinical transformation.
The epithelial-mesenchymal transition (EMT) and the development of a proangiogenic phenotype are thought to be the key events that promote invasive growth in epithelial malignancies. Additionally, it has been observed that activation of lymphangiogenesis enables rapid spread of cancer cells (Gutierrez-Miranda and Yaniv 2020). These systems rely on similar signaling cascades, and several studies have shown that there is significant molecular connectivity between them. Lymphatic vessels assist tumor spread by providing cancer cells with a route to access the lymph nodes and other places where they can spread (Pasquali et al. 2013). EMT promotes tumor cell migration by altering cell motility and promoting the degradation of the stromal layer and represents the driving force for cell migration to lymphatic vessels (Loh et al. 2019). The underlying molecular mechanisms of lymphangiogenesis and EMT in the early stages of the transition from intraepithelial development to invasive growth are of great interest because they could be used to prevent tumor spread.
IL-1RA is an antagonist of the IL-1 receptor, blocking IL-1-driven signaling to cells (Dayer 2002). In tumor progression, it usually acts as a tumor suppressor protein, preventing tumor proliferation and migration, angiogenesis, drug resistance, and other physiological and pathological activities through multiple mechanisms. In mice transplanted with IL-1β-secreting fibrosarcoma cells, sustained delivery of IL-1Ra reduces the inflammatory response and prevents tumor growth through the inhibition of angiogenesis (Bar et al. 2004). By blocking the IL-1α/VEGF signaling cascade, IL-1RA greatly reduced migration, proliferation, and tube formation of HUVEC cells, decreasing the likelihood of gastric carcinoma metastasis (Gong et al. 2018; Ma et al. 2017). Through downregulation of the IL-1α/PI3K/NF-κB cascade, IL-1RA may greatly reduce the metastatic capacity of colon cancer cells (Ma et al. 2017). The IL-1α-driven inflammatory activation of angiogenesis and lymphangiogenesis appears to provide a tumor microenvironment that favors lymph node metastasis through cross-talk with the IL-1R/M2-type macrophage axis (Watari et al. 2014). This study describes the role of IL-1a in the mediation of lymphangiogenesis. Whether IL-1RA, an antagonist of IL-1R, can regulate tumor-mediated lymphangiogenesis through this signaling pathway remains to be further explored. All of these findings suggest that IL-1RA may be a candidate biomarker for cancer. However, its function and mechanism of action in ESCC require investigation.
The development of bioinformatics technology provides a practical way to understand the molecular features and regulatory mechanisms of human disease processes in a higher dimension. The researchers mapped circRNA expression patterns using bioinformatics methods based on transcriptome sequencing data from a variety of solid cancer lesions, including esophageal squamous cell carcinoma; we also identified circlir, a molecule that can inhibit the progression of all six cancers (Wang et al. 2022). There are also studies that predict certain specific biomarkers by bioinformatics methods. The article found that CIREBCAR3 is a differential gene in esophageal cancer and analyzed the target molecule downstream of CIREBCAR3 by bioinformatics prediction; it has been successfully verified in a series of in vitro and in vivo experiments, providing another potential molecular pathway for targeted therapy of esophageal carcinoma (Xi et al. 2022). At the same time, NGS sequencing, protein–protein interaction network analysis, FunRich pathway analysis, and other technologies are also widely used in the diagnosis and treatment of cancer research (Pandey et al. 2023). In this study, we also explored IL-1RA-related signaling pathways and targets with the help of biological analysis.
This study investigated the expression and function of IL-1RA in ESCC as well as its underlying mechanism of action. Moreover, anakinra was investigated as an experimental therapy in animal models to assess its capacity as a possible targeted treatment.
Materials and methods
Tissue samples
Tissue samples were obtained at Fujian Medical University Union Hospital from 2009 and 2011. Overall, 100 human ESCC samples and their adjacent normal tissues were collected directly after surgical excision (Fuzhou, China). The samples were embedded and preserved in paraffin. The American Joint Committee on Cancer (AJCC) Eighth Edition of ESCC TNM staging was used to assess the clinical stage for each sample. This study received ethical approval (letter [2020] Fujian Medical University Union Hospital Ethical Examination No. (40)) from the Ethics Committee of Fujian Medical University.
Cell culture
A normal human esophageal epithelial cell line (CP-H031), as well as four ESCC cell lines (KYSE410, KYSE510, Eca109, and TE-1), was purchased from Guangzhou Cellcook Biotech Co., Ltd. All cell lines were grown in RPMI-1640 media (Gibco, Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS) (Gibco), at 37 °C in a humidified incubator with 5% CO2.
Immunohistochemical staining
Immunohistochemical (IHC) staining was done on FFPE (formalin-fixed paraffin-embedded) tissue Sects. (4–5 μm) cut from TMAs using rabbit anti-IL-1RA (1:1000, Abcam, UK; ab124692). IHC was performed strictly in line with the standard streptavidin–biotin-peroxidase complex method, and the staining signals were visually quantified using a scoring system from 0 to 9. The scores were obtained by multiplying the intensity of signals with the percentage of positive cells (signal: 0 = no signal, 1 = weak signal, 2 = intermediate signal, and 3 = strong signal; percentage: 0 = 0%, 1 ≤ 25%, 2 = 25–50%, and 3 ≥ 50%). Low and high expressions were defined as scores of < 6 and ≥ 6, respectively. Two pathologists independently examined each specimen.
RNA isolation, cDNA construction, and qRT-PCR evaluation
Total RNA was extracted from cells and tissues using TRizol. An RT Reagent kit (Takara, China) was used to reverse-transcribe 1 mg of RNA to cDNA. SYBR Premix Ex Taq® kit (Takara, Shiga, Japan) was used to conduct quantitative PCR on an Mx3000P® qPCR system (Agilent Technologies, USA) with gene-specific primers IL-1RA-F (5′-TTCCTGTTCCATTCAGAGACGAT-3′) and IL-1RA-R (5′-CCAGATTCTGAAGGCTTGCAT-3′). GAPDH-F (5′-GCGGGGCTCCA GAACATCAT-3′), with the GAPDH-R (5′-CCAGCCCCAGCGTCAAGGTG-3′) primer, was used as a housekeeping gene for the normalizing of the expression data. Relative expression was calculated using the 2−ΔΔCt formula. Each experiment was conducted independently trice, and the mean values were taken.
Western blot analysis
Cells and tissues were lysed with western/IP cell lysis buffer (Beyotime, China) containing PMSF (Amresco, USA) for 30 min on ice. The lysates were centrifuged at 12,000 g for 10 min at 4 °C). The supernatants containing the total protein were placed in a fresh Eppendorf tube and the protein concentrations measured using a BCA kit (Thermo Scientific, USA). The proteins were separated on 10% SDS-PAGE and transferred to 0.45-μm polyvinylidene difluoride (PVDF) membranes (Amersham Hybond®, GE Healthcare, München, Germany). Bovine serum albumin (0.5%) (Amresco) was used as a blocking reagent, and the blots were incubated overnight at 4 °C with specific antibodies (supplementary file).
The membranes were then washed three times with Tris-buffered saline containing 0.1% Tween-20 detergent (TBST) for 20 min before treatment with secondary antibodies for 1 h at ambient temp. The bands were visualized using enhanced chemiluminescence (Lulong Biotech, China).
The following primary antibodies were employed: rabbit anti-IL-1RA antibody (1:1000, Abcam, ab124692), mouse anti-β-actin (1:1000, Abcam, ab8226), rabbit anti-MMP2 (1:1000, Abcam, ab97779), rabbit anti-MMP9 (1:1000, Abcam, ab228402), rabbit anti-MMP3(1:1000, Abcam, ab52915), rabbit anti-N-cadherin (1:1000; Cell Signaling Technology), rabbit anti-vimentin (1:1000; Cell Signaling Technology), and rabbit anti-N-cadherin (1:1000; Cell Signaling Technology), at 4 °C overnight.
VEGF measurement by ELISA
Enzyme-linked immunosorbent assays (ELISAs) were performed with an ELISA kit (ab222510, Abcam) to measure the VEGF in the samples. VEGF concentrations were determined in the culture supernatants of stable IL-1RA-overexpressing Eca109 cells and KYSE410 cells using the manufacturer’s provided protocol.
Lymphatic vessel formation and invasion assay
Approximately 1 × 104 human lymphatic endothelial cells (hLECs) were seeded in each well of 96-well plates coated with ice-thawed 50 μl of Matrix® Growth Factor Reduced (GFR) Basement Membrane Matrix. These cells were co-cultured with the supernatants derived from KYSE410 and Eca109 cells stably overexpressing IL-1RA for 24 h. The capillary network length in the tubes was measured in 10 random microscopic fields using computer-assisted microscopy.
Approximately 4 × 104 cells in serum-free medium (SFM) containing 20% FBS (as chemoattractant) were placed in the lower chamber of a Transwell apparatus coated with Matrigel (BD Bioscience) and incubated at 37 °C with 5% CO2. Non-migrated cells were removed from the upper chamber using cotton swabs, and the cells that had settled on the filter’s underside were stained with 0.1% crystal violet in 20% methanol for 10 min before assessment and imaging with a microscope (Qimaging Micropublisher Ver-5.0 RTV, Olympus, Japan).
Clonogenic assay
To check for the formation of colonies, 500 cells were seeded into a 60-mm plate. In this assay, RPMI-1640 + 10% FBS was employed. The culture plates were kept in an incubator (37 °C/5% CO2) for 2 weeks. Colonies were stained with crystal violet for 5 min, and colonies > 50 cells were counted and imaged.
Cell migration assay
To analyze cell migration, 4 × 104 cells in SFM were seeded within the upper chamber of a Transwell insert (8-mm pore size; BD Bioscience) together with 20% FBS (as a chemoattractant) in the lower chamber and incubated for 24 h.
The non-migrated cells were removed with cotton swabs, and the lower surface of the filter was stained with 0.1% crystal violet in 20% methanol for 10 min before counting and imaging of the cells.
Transcriptome analysis for DEG identification
Transcriptome sequencing was done to compare the gene expression profiles of Eca109 cells overexpressing IL-1RA and a control group. Following the manufacturer’s recommended procedures, RNA sequencing was performed on an Illumina platform (Novogene Co., LTD, China). The indexed reference genome was aligned with the clean reads using the HISAT v2.0.5 pipeline with the default settings.
The featureCounts (https://rnnh.github.io/bioinfo-notebook/docs/featureCounts.html) program counted the total number of readings that matched each gene. Differentially expressed genes (DEGs) between healthy and tumor tissues were determined using the DESeq2 package in R Ver-1.16.1 pipeline together with Benjamini–Hochberg adjusted P-values. P-values = 0.01 and a log2 fold change = 2.0 were used as the cutoffs for differential expression significance. A TruSeq PE Cluster kit v3-cBotHS was used for the sample clustering, executed on a cBot Cluster Generation System (Illumina). Hierarchical clustering of significant DEGs was performed.
Bioinformatics analysis
The clusterProfiler R-package (https://bioconductor.org/packages/release/bioc/html/cluster Profiler.html) was used to conduct Gene Ontology (GO) annotation for the DEGs. This allowed us to understand the biological roles played by each gene. The KEGG database (http://www.genome.jp/kegg/) was used to study the correlation between pathways and their activation in both healthy and pathological states.
Animal experiments
Anakinra, an interleukin 1 receptor antagonist, was compared to the IL-1RA gene in nude mice by subcutaneous implantation of cells and intravenous injection of the drug. Four-week-old female BALB/c mice were randomly divided into two groups. Four mice received intravenous injections of 2 × 106 Eca109 cells overexpressing IL-1RA in 0.2-ml PBS. After four weeks, the mice were sacrificed and their organs were harvested. The presence of lung and liver metastases was examined under a dissecting microscope.
The following formula was used to calculate the tumor volumes:
The weights of the mice were recorded daily, and tumor weights were measured following the surgical removal of the malignant tissue. Female BALB/c nude murine were subcutaneously injected with Eca109 cells. Then, anakinra (2 mg/kg) was injected at the inoculation site once a day for 4 weeks as part of the therapeutic experiment. The Institutional Animal Care and Use Committee at Fujian Medical University approved all animal experiments (FJMU IACUC 2021–0486).
Statistical analysis
Statistical analyses were performed using SPSS version 23.0 (IBM Corp., USA) for Windows and GraphPad Prism 7 (San Diego, CA, USA) software. All of the biological replicates were performed at least 3 times in all of our experiments. And all data used for the analysis were expressed as the mean ± SD from three independent assays. Clinicopathological characteristics were analyzed by the chi-square test. Survival curves were evaluated using the Kaplan–Meier method. All P-values were determined from two-tailed tests, and differences with a P-value < 0.05 were considered statistically significant.
Results
Downregulation of IL-1RA in human ESCC tissues and cell lines
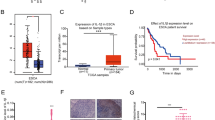
The mRNA sequencing data acquired from the GTEx database revealed that IL-1RA was downregulated in ESCC tissues in comparison to healthy esophageal tissues (P < 0.0001, Fig. 1A–C), which was consistent with the data obtained from mRNA sequencing of 5 ESCC patients (Supplementary Fig. 1). IL-1RA, a secreted protein with a length of 177 amino acids, was upregulated in esophageal epithelial tissue, as depicted in Fig. 1D–F. We used immunohistochemical staining to assess the expression of IL-1RA in 100 matched ESCC specimens and corresponding non-tumor tissues to ascertain whether IL-1RA contributed to the regulation of human ESCC tumorigenesis. Following that, the link between IL-1RA expression and clinical and pathological factors was evaluated (Table 1). As shown in Fig. 2C and D, IL-1RA was downregulated in human ESCC tissue and was correlated with both advanced clinical stage and lymphatic metastasis. Additionally, compared with normal esophageal epithelial cells CP-H031, IL-1RA was markedly downregulated in the human ESCC cell lines KYSE510, KYSE410, Eca109, and TE-1, as shown in Fig. 2A and B. These findings suggest that the lack of IL-1RA may influence the onset and progression of ESCC.
IL-1RA expression in ESCC. A Hierarchical clustering heatmap showing the expression of mRNAs in ESCC tissues and adjacent non-tumor tissues. B Volcano plot showing the expression of mRNAs in 74 paired ESCC tissues and adjacent non-tumor tissues. C IL-1RA mRNA expression in tumor and adjacent non-tumor tissues. D Location of the IL-1RA gene on the chromosome. E The expression of IL-1RA in different human tissues. F Protein structure of IL-1RA. Note: N normal tissue, T tumor tissue
Expression of IL-1RA in ESCC. A IL-1RA protein levels in ESCC cell lines and a normal esophageal cell line (CP H031) were assessed by western blot analyses. B IL-1RA mRNA levels in ESCC cell lines and a normal esophageal cell line (CP H031) were measured by qRT-PCR. *P < 0.05. C IL-1RA immunohistochemical score in ESCC tissues according to four staining intensity classes. D Monofactor analysis of the relationship between IL-1RA expression and lymph node metastasis of esophageal squamous cell carcinoma. Note: IL-1RN interleukin 1 receptor antagonist
IL-1RA inhibited ESCC proliferation, migration, and lymphangiogenesis
To examine the impact of IL-1RA overexpression on ESCC cells, IL-1RA-overexpressing KYSE410 and Eca109 cells were developed. IL-1RA overexpression was confirmed by western blotting and qRT-PCR. Higher levels of IL-1RA were observed in cells transfected with the pIL-1RA vector than in cells transfected with the pCDH empty vector (Fig. 3A and B).
IL-1RA expression regulated ESCC. A Western blot analysis confirmed the overexpression of IL-1RA in KYSE410 and Eca109 cells following stable transfection. B qRT-PCR analysis confirmed the overexpression of IL-1RA in KYSE410 and Eca109 cells following stable transfection. C Hierarchical clustering heatmap showing mRNA expression in ESCC cells and differential expression of IL-1RA. D Volcano plot showing mRNA expression profiles in ESCC cells with differential expression of IL-1RA. E Gene set enrichment analysis suggesting the influence of IL-1RA expression on the biological function of ESCC cells. F KEGG analysis showing signaling pathways affected by altered IL-1RA expression
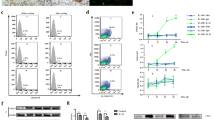
The effects of IL-1RA overexpression on ESCC cell proliferation were then investigated. The growth of KYSE410 and Eca109 cells was significantly reduced by IL-1RA overexpression, shown by colony-forming assays (Fig. 4A). Transwell assays were used to confirm whether IL-1RA overexpression reduced ESCC cell migration, showing that invasion was significantly reduced in cells overexpressing IL-1RA (Fig. 4B). Since IL-1RA expression was linked to lymphatic metastases in all ESCC cases, we hypothesized that IL-1RA expression would decrease lymphangiogenesis and migration in ESCC. Lymphocyte formation and migration experiments showed that the culture supernatants of IL-1RA-overexpressing ESCC cells significantly inhibited lymphangiogenesis and migration (Fig. 4C and D).
IL-1RA inhibited the proliferation and migration of ESCC cells and lymphatic endothelial cells in vitro. A Upregulated IL-1RA expression significantly decreased the ESCC colony numbers. B Upregulated IL-1RA expression reduced ESCC cell migration. C Upregulated IL-1RA expression reduced the ring-forming ability of lymphatic endothelial cells. D Upregulated IL-1RA expression reduced migration of lymphatic endothelial cells
In the in vivo experiments, we found that the growth rate of IL-1RA-overexpressing Eca109 subcutaneous xenografts was significantly slower than that of the control Eca109 p-CDH tumors (Fig. 4A–F). Immunohistochemical staining of the lymphatic vessel marker VEGFR3 showed that the number of lymphatic vessels in Eca109 P-IL-1RA tumors was considerably decreased compared with controls (Fig. 4G). Furthermore, the number of Eca109 P-IL-1RA metastases in the lungs was significantly lower compared with the controls (Fig. 4H–I). This implies that IL-1RA expression inhibited ESCC cell growth, invasion, lymphangiogenesis, and metastasis (Fig. 5).
IL-1RA inhibited the proliferation and migration of ESCC cells and lymphatic endothelial cells in vivo. A Tumor tissue from nude mice subcutaneously injected with Eca109 cells. B Weight change of the mice after subcutaneous injection of Eca109 cells. C Tumor size change after subcutaneous injection of Eca109 cells. D Tumor weights in 4 weeks after subcutaneous injection of Eca109 cells. E VEGFR3 expression in mouse tumor tissue shown by immunohistochemistry. F VEGFR3 expression in relation to IL-1RA expression. G Liver and lung tissue in xenograft mouse models. H Lung metastases shown by HE staining. I The analyzed of data for lung cancer nests
In the anakinra treatment experiment, we also found that the tumor volume, mass, and the number of metastatic lesions in the treated group were considerably reduced relative to the saline group (Fig. 6).
Anakinra inhibited the proliferation and migration of ESCC cells and lymphatic endothelial cells in vivo. A Mouse xenograft tumor tissue after anakinra treatment. B Body weight change of xenograft mice after anakinra treatment. C Tumor volume change after anakinra treatment. D Tumor weights in four weeks after anakinra treatment. E VEGFR3 expression in mouse tumors after anakinra treatment, shown by immunohistochemistry. F Lungs and livers in mice after anakinra treatment. G Lung metastases after anakinra treatment, shown by HE staining
Impact of IL-1RA on the ESCC transcriptome
High-throughput mRNA sequencing was then used to analyze the mRNA expression profiles in IL-1RA-overexpressing cells for investigating the impact of IL-1RA expression on the ESCC cell transcriptome. Gene expression profiles were shown using hierarchical clustering (Fig. 3C). We observed 4135 DEGs, of which 2476 were upregulated and 1659 downregulated (with a fold change of ≥ 2.0, P-adj < 0.05); these included members of the VEGF and MMP families, which are known to have a close link with lymph node metastasis. Volcano plots, which show fold change in gene expression (x-axis) and importance of the variation in gene expression between pools (y-axis), were created to give insight into the differential gene expression (Fig. 3D). The well-known NF-κB signaling cascade and the pathways linked to protein processing, the cell cycle, and the inflammation network were found to be the most significantly enriched in the KEGG analysis (Fig. 3E and F). According to the GO analysis, the DEGs were observed in all three GO categories, namely, biological process, cellular component, and molecular function.
IL-1RA was associated with MMP9 expression and EMT induction
Cancer cells acquire motile and invasive abilities through activation of the EMT, one of the crucial steps in the metastatic process. Hence, we investigated whether IL-1RA contributed to the modulation of EMT in ESCC cells. As shown in Fig. 7, overexpression of IL-1RA in KYSE410 and Eca109 cells significantly reduced MMP9, N-cadherin, and vimentin expression, while it increased E-cadherin expression (Fig. 7A). Moreover, forced expression of MMP9 in IL-1RA-overexpressing KYSE410 and Eca109 cells reversed this change (Fig. 7B). These results suggest that reduced expression of IL-1RA is linked with the enhancement of ESCC cell migration, which is closely correlated with MMP9-mediated activation of the EMT.
IL-1RA inhibited EMT progression via MMP9 in ESCC together with the expression and secretion of VEGF-C via the PI3K/NF-κB pathway. A MMP2/MMP3/MMP9 expression in ESCC cells transfected with p-CDH and p-IL-1RA shown by western blotting. B E-cadherin, N-cadherin, and vimentin expression after upregulation of IL-1RA and MMP9 supplementation, shown by western blotting. C Expression of VEGF-A/B/C/D, COX-2, and HIF-α, shown by ELISA. D Stimulation of the PI3K/NF-κB pathway by IL-1RA overexpression, shown by western blotting
IL-1RA inhibited the transcription, expression, and secretion of VEGF-C via PI3K/NF-κB signaling cascade
VEGF-C is a powerful promoter of lymphatic vessel self-generation. The transcriptomic analysis suggested that IL-1RA expression might reduce VEGF-C transcription. We explored the impact of IL-1RA overexpression on the expression and secretion of the VEGF family by ELISA, and the results suggested that IL-1RA significantly attenuated VEGF-C expression (Fig. 7C). Furthermore, the bioinformatics analysis indicated that IL-1RA could inhibit the PI3K/N-FKB signaling pathway, which was confirmed by western blotting showing that overexpression of IL-1RA can significantly inhibit PI3K expression and phosphorylation of NF-κB in ESCC cells (Fig. 7D).
Discussion
Although lymph node metastasis is known to play a major role in the poor prognosis of ESCC, its underlying mechanism is still unknown. We found that IL-1RA was markedly reduced in ESCC tissues through 2-D-protein electrophoresis, mass spectrometry analysis (Yu et al. 2017), high-throughput mRNA sequencing, and TCGA database analysis. Furthermore, there was a correlation between lymphatic metastasis and tumor pathological stage in ESCC patients, as well as with the degree of IL-1RA expression. Moreover, IL-1RA expression was significantly lower in ESCC cell lines compared with the normal CP-H031 esophageal cell line. Thus, downregulation of IL-1RA was strongly linked to ESCC lymph node metastases and a poor prognosis in ESCC patients.
Cancer-related phenotypes were investigated in ESCC cells to understand the function of IL-1RA. It was found that IL-1RA inhibited ESCC cell proliferation and invasion, suggesting that IL-1RA inhibits ESCC carcinogenesis and may serve as both an important diagnostic biomarker and a potential treatment target for this malignancy. Recent research has demonstrated that IL-1RA inhibits tumor growth by reducing angiogenesis. In ESCC, the main reason for the high malignancy of the tumor is lymph node metastasis. However, the correlation between IL-1RA and lymph node metastasis remains elusive.
The dissemination of cancer is correlated with the EMT, which accelerates tumor development (Mitschke et al. 2019). Cancer cells lose cell–cell junctions, acquire mesenchymal properties, and develop a degradable matrix layer during the EMT, which increases their migratory and invasive properties (Thierauf et al. 2017; Wei et al. 2021). This study found that when IL-1RA was overexpressed in ESCC-KYSE410 and Eca109 cells, the expression levels of MMP9, vimentin, and N-cadherin were decreased while those of E-cadherin were raised. MMP2 and MMP9 are documented to be key factors that stimulate EMT-induced tumor progression (Liu et al. 2019). It has been reported that MMP2 and MMP9 regulate EMT marker proteins to degrade the ECM, thus reducing cell adhesion and allowing cells to adopt a mesenchymal phenotype and to move from the basement membrane (Yang et al. 2018b; Zhang and Weinberg 2018). It has been demonstrated that the MMP-activated EMT promotes the development of multiple cancers (Che et al. 2019; Li et al. 2019; Ma et al. 2018). We found that downregulation of IL-1RA expression caused changes in the expression of MMP9, N-cadherin, vimentin, and E-cadherin, thereby triggering the EMT, which was also confirmed by the MMP9 rescue experiment. According to these results, IL-1RA can inhibit the progression of the EMT by regulating transcription and expression of MMP9 to reduce ESCC metastatic potential.
The key activators of angiogenesis and lymphangiogenesis are VEGFs (Matkar et al. 2017). Primary targets for the pathological management of angiogenic and lymphangiogenic signaling also include VEGFs and their receptors (VEGFRs) (Zhang et al. 2020). While VEGF-C is involved in lymphangiogenesis, VEGF-A primarily controls angiogenesis. VEGF-C promotes the growth of tumors through a number of pathways. Moreover, VEGFC/VEGFR-3 autocrine signaling is associated with both tumor invasion and proliferation, as well as lymph node metastasis (Wang et al. 2010). In ESCC, lymph node metastases and a poor prognosis are significantly linked with VEGF-C expression (Lin et al. 2015). Even in patients in the Tis and T1 stages, VEGF-C can promote the conversion of primitive capillary lymphocytes in tumor tissue into capillary lymphatic vessels, promote the formation of lymphatic vessels, and provide an essential transmission path for lymph node metastasis of ESCC (Smyth et al. 2017).
Our findings confirm the pivotal role of VEGF-C in IL-1RA-regulated lymphangiogenesis. This study showed that the change in VEGF-C level was due to reduced transcription. Many reports have implicated IL-1RA in the inhibition of PI3K/NF-κB signaling. Additionally, the NF-κB transcription factor controls VEGF-C transcription (Sainz-Jaspeado and Claesson-Welsh 2018; Tacconi et al. 2019). Therefore, this study suggested that IL-1RA may inhibit PI3K/NF-κB signaling and thus inhibit the transcription of VEGF-C, leading to a reduction in ESCC lymphangiogenesis and lymph node metastasis. However, the effect of IL-1RA in lymphangiogenesis in vivo and the precise mechanism by which IL-1RA influences VEGF-C transcription remain unknown and require further investigation.
Anakinra is an IL-1R antagonist that can be used effectively in the treatment of many human diseases, and its safety has been confirmed (Fitzgerald et al. 2005; Venugopal et al. 2021). There are few adverse effects, making it a safe alternative drug for cancer treatment. It has been reported that anti-IL-1β drugs can block TNF-α expression induced by IL-1β in lung cancer to obtain an auxiliary anti-tumor effect (Ridker et al. 2017). However, the effectiveness of IL-1R antagonists as adjuvant therapy in ESCC remains elusive. Using the standard administration method of 2 mg/kg, once every 2 days and subcutaneous injection 1 cm from the tumor, it was found that, compared with the controls, ESCC xenograft tumors were significantly reduced in nude mice, shown by reductions in the tumor growth rate, tumor volume, and the formation of lymphatic vessels in the tumor. These data demonstrated that anakinra may be a useful treatment for ESCC. Although we have explored the regulatory relationship between IL-1RA and pre-lymphangiogenesis initially, the transcriptional regulatory sites of the PI3K/NF-κB signaling pathway remain to be further explored. And the effective drug concentration and side effects of anakinra need to be further studied.
Conclusions
This study showed that IL-1RA is downregulated in ESCC and IL-1RA overexpression suppresses tumor lymphatic metastasis by targeting MMP9 and VEGF-C. Furthermore, the downregulation of IL-1RA can activity of the EMT programs and subsequent malignant ESCC behavior. Notably, the IL-1R antagonist anakinra was found to be effective in inhibiting ESCC proliferation as well as lymph node metastasis. Taken together, this study examined the function and mechanism of IL-1RA in ESCC lymph node metastasis, which may offer a scientific foundation for the clinical diagnosis, treatment, and improved prognosis of ESCC.
Data Availability
The data are available from the corresponding author on reasonable request.
Change history
11 May 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s10142-024-01368-1
References
Bar D, Apte R, Voronov E, Dinarello C, Cohen S (2004) A continuous delivery system of IL-1 receptor antagonist reduces angiogenesis and inhibits tumor development. FASEB J 18:161–163
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Che Y, Li Y, Zheng F, Zou K, Li Z, Chen M, Hu S, Tian C, Yu W, Guo W, Luo M, Deng W, Zou L (2019) TRIP4 promotes tumor growth and metastasis and regulates radiosensitivity of cervical cancer by activating MAPK, PI3K/AKT, and hTERT signaling. Cancer Lett 452:1–13
Dayer J (2002) Evidence for the biological modulation of IL-1 activity: the role of IL-1Ra. Clin Exp Rheumatol 20:S14-20
Fatehi Hassanabad A, Chehade R, Breadner D, Raphael J (2020) Esophageal carcinoma: towards targeted therapies. Cell Oncol (dordr) 43:195–209
Fitzgerald AA, Leclercq SA, Yan A, Homik JE, Dinarello CA (2005) Rapid responses to anakinra in patients with refractory adult-onset Still’s disease. Arthritis Rheum 52:1794–1803
Gao YB, Chen ZL, Li JG, Hu XD, Shi XJ, Sun ZM, Zhang F, Zhao ZR, Li ZT, Liu ZY, Zhao YD, Sun J, Zhou CC, Yao R, Wang SY, Wang P, Sun N, Zhang BH, Dong JS, Yu Y, Luo M, Feng XL, Shi SS, Zhou F, Tan FW, Qiu B, Li N, Shao K, Zhang LJ, Zhang LJ, Xue Q, Gao SG, He J (2014) Genetic landscape of esophageal squamous cell carcinoma. Nat Genet 46:1097–1102
Gong Z, Ma J, Su H, Guo T, Cai H, Chen Q, Zhao X, Qi J, Du J (2018) Interleukin-1 receptor antagonist inhibits angiogenesis in gastric cancer. Int J Clin Oncol 23:659–670
Gutierrez-Miranda L, Yaniv K (2020) Cellular origins of the lymphatic endothelium: implications for cancer lymphangiogenesis. Front Physiol 11:577584
Hao JJ, Lin DC, Dinh HQ, Mayakonda A, Jiang YY, Chang C, Jiang Y, Lu CC, Shi ZZ, Xu X, Zhang Y, Cai Y, Wang JW, Zhan QM, Wei WQ, Berman BP, Wang MR, Koeffler HP (2016) Spatial intratumoral heterogeneity and temporal clonal evolution in esophageal squamous cell carcinoma. Nat Genet 48:1500–1507
Li Y, Wang T, Sun Y, Huang T, Li C, Fu Y, Li Y, Li C (2019) p53-mediated PI3K/AKT/mTOR pathway played a role in Ptox(Dpt)-induced EMT inhibition in liver cancer cell lines. Oxid Med Cell Longev 2019:2531493
Lin C, Song L, Liu A, Gong H, Lin X, Wu J, Li M, Li J (2015) Overexpression of AKIP1 promotes angiogenesis and lymphangiogenesis in human esophageal squamous cell carcinoma. Oncogene 34:384–393
Liu SQ, Xu CY, Wu WH, Fu ZH, He SW, Qin MB, Huang JA (2019) Sphingosine kinase 1 promotes the metastasis of colorectal cancer by inducing the epithelialmesenchymal transition mediated by the FAK/AKT/MMPs axis. Int J Oncol 54:41–52
Loh CY, Chai JY, Tang TF, Wong WF, Sethi G, Shanmugam MK, Chong PP, Looi CY (2019) The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells 8(10):1118
Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D (2016) Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 27:v50–v57
Ma J, Sun X, Guo T, Su H, Chen Q, Gong Z, Qi J, Zhao X (2017) Interleukin-1 receptor antagonist inhibits angiogenesis via blockage IL-1alpha/PI3K/NF-kappabeta pathway in human colon cancer cell. Cancer Manag Res 9:481–493
Ma YS, Wu ZJ, Bai RZ, Dong H, Xie BX, Wu XH, Hang XS, Liu AN, Jiang XH, Wang GR, Jiang JJ, Xu WH, Chen XP, Tan GH, Fu D, Liu JB, Liu Q (2018) DRR1 promotes glioblastoma cell invasion and epithelial-mesenchymal transition via regulating AKT activation. Cancer Lett 423:86–94
Matkar PN, Ariyagunarajah R, Leong-Poi H, Singh KK (2017) Friends turned foes: angiogenic growth factors beyond angiogenesis. Biomolecules 7(4):74
Mitschke J, Burk UC, Reinheckel T (2019) The role of proteases in epithelial-to-mesenchymal cell transitions in cancer. Cancer Metastasis Rev 38:431–444
Pandey P, Suyal G, Aprajita, Pasbola K, Sharma R (2023) NGS-based profiling identifies miRNAs and pathways dysregulated in cisplatin-resistant esophageal cancer cells. Funct Integr Genomics 23(2):111
Pasquali S, van der Ploeg AP, Mocellin S, Stretch JR, Thompson JF, Scolyer RA (2013) Lymphatic biomarkers in primary melanomas as predictors of regional lymph node metastasis and patient outcomes. Pigment Cell Melanoma Res 26:326–337
Rice TW, Patil DT, Blackstone EH (2017) 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 6:119–130
Ridker PM, MacFadyen JG, Thuren T, Everett BM (2017) Libby P and Glynn RJ Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. The Lancet 390:1833–1842
Sainz-Jaspeado M, Claesson-Welsh L (2018) Cytokines regulating lymphangiogenesis. Curr Opin Immunol 53:58–63
Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, Lordick F, Kim SB, Tajika M, Kim HT, Lockhart AC, Arkenau HT, El-Hajbi F, Gupta M, Pfeiffer P, Liu Q, Lunceford J, Kang SP, Bhagia P, Kato K (2019) Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 study. JAMA Oncol 5:546–550
Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, Cunningham D (2017) Oesophageal cancer. Nat Rev Dis Primers 3:17048
Song Y, Li L, Ou Y, Gao Z, Li E, Li X, Zhang W, Wang J, Xu L, Zhou Y, Ma X, Liu L, Zhao Z, Huang X, Fan J, Dong L, Chen G, Ma L, Yang J, Chen L, He M, Li M, Zhuang X, Huang K, Qiu K, Yin G, Guo G, Feng Q, Chen P, Wu Z, Wu J, Ma L, Zhao J, Luo L, Fu M, Xu B, Chen B, Li Y, Tong T, Wang M, Liu Z, Lin D, Zhang X, Yang H, Wang J, Zhan Q (2014) Identification of genomic alterations in oesophageal squamous cell cancer. Nature 509:91–95
Tacconi C, Ungaro F, Correale C, Arena V, Massimino L, Detmar M, Spinelli A, Carvello M, Mazzone M, Oliveira AI, Rubbino F, Garlatti V, Spanò S, Lugli E, Colombo FS, Malesci A, Peyrin-Biroulet L, Vetrano S, Danese S, D’Alessio S (2019) Activation of the VEGFC/VEGFR3 pathway induces tumor immune escape in colorectal cancer. Cancer Res 79:4196–4210
Thierauf J, Veit JA, Hess J (2017) Epithelial-to-mesenchymal transition in the pathogenesis and therapy of head and neck cancer. Cancers (Basel) 9:76
Venugopal J, Wang J, Mawri J, Guo C, Eitzman D (2021) Interleukin-1 receptor inhibition reduces stroke size in a murine model of sickle cell disease. Haematologica 106(9):2469–2477
Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Lüthi U, Barberis A, Benjamin LE, Mäkinen T, Nobes CD, Adams RH (2010) Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465:483–486
Wang C, Liu WR, Tan S, Zhou JK, Xu X, Ming Y, Cheng J, Li J, Zeng Z, Zuo Y, He J, Peng Y, Li W (2022) Characterization of distinct circular RNA signatures in solid tumors. Mol Cancer 21(1):63
Watari K, Shibata T, Kawahara A, Sata K, Nabeshima H, Shinoda A, Abe H, Azuma K, Murakami Y, Izumi H, Takahashi T, Kage M, Kuwano M, Ono M (2014) Tumor-derived interleukin-1 promotes lymphangiogenesis and lymph node metastasis through M2-type macrophages. PLoS ONE 9(6):e99568
Wei S, Sun S, Zhou X, Zhang C, Li X, Dai S, Wang Y, Zhao L, Shan B (2021) SNHG5 inhibits the progression of EMT through the ubiquitin-degradation of MTA2 in oesophageal cancer. Carcinogenesis 42(2):315–326
Xi Y, Shen Y, Wu D, Zhang J, Lin C, Wang L, Yu C, Yu B, Shen W (2022) CircBCAR3 accelerates esophageal cancer tumorigenesis and metastasis via sponging miR-27a-3p. Mol Cancer 21(1):145
Yang YM, Hong P, Xu WW, He QY, Li B (2018a) Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol 36:2796–2803
Yang L, Shang Z, Long S, Wang N, Shan G, Zhang R (2018b) Roles of genetic and microenvironmental factors in cancer epithelial-to-mesenchymal transition and therapeutic implication. Exp Cell Res 370:190–197
Yu SB, Gao Q, Lin WW, Kang MQ (2017) Proteomic analysis indicates the importance of TPM3 in esophageal squamous cell carcinoma invasion and metastasis. Mol Med Rep 15:1236–1242
Zhang Y, Weinberg RA (2018) Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Med 12:361–373
Zhang X, Zhang Y, Jia Y, Qin T, Zhang C, Li Y, Huang C, Liu Z, Wang J, Li K (2020) Bevacizumab promotes active biological behaviors of human umbilical vein endothelial cells by activating TGFbeta1 pathways via off-VEGF signaling. Cancer Biol Med 17:418–432
Funding
This study was supported by National Natural Science Foundation of China (grant numbers 81773129 and 82070499); Startup Fund for Scientific Research, Fujian Medical University (grant number 2021QH1021); and Fujian Provincial Department of Education Young and Middle-aged Teachers Program (grant number JAT201196).
Author information
Authors and Affiliations
Contributions
ZMS and MQK designed this study. ZMS and WGZ performed all the experiments. PPZ, SCC, and ZMS collected clinical data. ZMS, PPZ, and SCC interpreted the data. SC, HX, FL, MQK, and SCC offered technical support. ZMS and MQK wrote the manuscript. All authors polished the paper.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Ethical Boards of Fujian Medical University Union Hospital (grant number FJMU IACUC2021-0486). Written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s10142-024-01368-1"
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shen, Z., Zhang, P., Zhang, W. et al. RETRACTED ARTICLE: IL-1RA inhibits esophageal carcinogenesis and lymphangiogenesis via downregulating VEGF-C and MMP9. Funct Integr Genomics 23, 164 (2023). https://doi.org/10.1007/s10142-023-01049-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10142-023-01049-5