Abstract
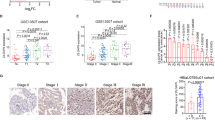
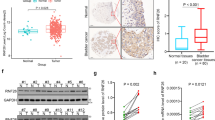
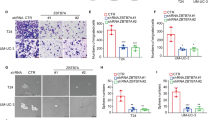
In bladder cancer patients, metastasis after surgical resection and serious adverse reactions brought by cisplatin-based systemic chemotherapy make it urgent to explore novel therapeutic methods for improving the clinical outcomes of patients with unsuccessful first-line chemotherapy and disease progression. In this study, GBX2 has been recognized as a differentially expressed transcriptional factor between bladder cases with response to treatment and progressive disease based on online expression profile analysis. Higher GBX2 expression was correlated with poorer OS, DSS, and PFS in bladder cancer patients. GBX2 co-expressed genes were enriched in ECM regulation. ITGA5 was positively correlated with GBX2. GBX2 and ITGA5 were notably elevated in bladder cancer cells. GBX2 and ITGA5 similarly affected bladder cancer cell phenotypes via facilitating cell viability, migration, and invasion. By binding to the promoter region of ITGA5, GBX2 activated ITGA5 transcription, upregulating ITGA5 expression. In bladder cancer cells co-transfected with sh-GBX2 and ITGA5 oe, the inhibitory effects of GBX2 knockdown on bladder cancer cell malignant behaviors were partially eliminated by ITGA5 overexpression. In conclusion, GBX2 and ITGA5 serve as oncogenic factors, promoting the viability, migration, and invasion of bladder cancer cells. GBX2 exerts its functions by targeting the ITGA5 promoter region to activate ITGA5 transcription.







Similar content being viewed by others
References
Ahmadi H, Duddalwar V, Daneshmand S (2021) Diagnosis and staging of bladder cancer. Hematol Oncol Clin North Am 35(3):531–541
Bray F et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Chen X, Cheng P, Hu C (2021) LncRNA FEZF1-AS1 accelerates the migration and invasion of laryngeal squamous cell carcinoma cells through miR-4497 targeting GBX2. Eur Arch Otorhinolaryngol 278(5):1523–1535
Cookson MS et al (1997) The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol 158(1):62–67
Cui X et al (2016) miR-130b, an onco-miRNA in bladder cancer, is directly regulated by NF-kappaB and sustains NF-kappaB activation by decreasing Cylindromatosis expression. Oncotarget 7(30):48547–48561
Dash A et al (2006) Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 107(3):506–513
Davies B et al (1993) Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res 53(22):5365–5369
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2(3):161–174
Gao AC, Lou W, Isaacs JT (1998) Down-regulation of homeobox gene GBX2 expression inhibits human prostate cancer clonogenic ability and tumorigenicity. Cancer Res 58(7):1391–1394
Gao AC, Lou W, Isaacs JT (2000) Enhanced GBX2 expression stimulates growth of human prostate cancer cells via transcriptional up-regulation of the interleukin 6 gene. Clin Cancer Res 6(2):493–497
Gong C et al (2016) miR-17 inhibits ovarian cancer cell peritoneal metastasis by targeting ITGA5 and ITGB1. Oncol Rep 36(4):2177–2183
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Helin K, Dhanak D (2013) Chromatin proteins and modifications as drug targets. Nature 502(7472):480–488
Jeyapala R et al (2019) GBX2 methylation is a novel prognostic biomarker and improves prediction of biochemical recurrence among patients with prostate cancer negative for intraductal carcinoma and cribriform architecture. Eur Urol Oncol 2(3):231–238
Kader AK et al (2007) Matrix metalloproteinase polymorphisms are associated with bladder cancer invasiveness. Clin Cancer Res 13(9):2614–2620
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6(5):392–401
Lee TI, Young RA (2013) Transcriptional regulation and its misregulation in disease. Cell 152(6):1237–1251
Marelli UK et al (2013) Tumor targeting via integrin ligands. Front Oncol 3:222
Martens JH, Stunnenberg HG (2010) The molecular signature of oncofusion proteins in acute myeloid leukemia. FEBS Lett 584(12):2662–2669
Milowsky MI et al (2016) Guideline on muscle-invasive and metastatic bladder cancer (European Association of Urology Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol 34(16):1945–1952
Miyake M et al (2017) Diagnostic and prognostic role of urinary collagens in primary human bladder cancer. Cancer Sci 108(11):2221–2228
Musavi Shenas SMH et al (2017) SiRNA-mediated silencing of Snail-1 induces apoptosis and alters micro RNA expression in human urinary bladder cancer cell line. Artif Cells Nanomed. Biotechnol 45(5):969–974
Ploussard G et al (2014) Conditional survival after radical cystectomy for bladder cancer: evidence for a patient changing risk profile over time. Eur Urol 66(2):361–370
Riester M et al (2012) Combination of a novel gene expression signature with a clinical nomogram improves the prediction of survival in high-risk bladder cancer. Clin Cancer Res 18(5):1323–1333
Song T et al (2015) Decrement of miR-199a-5p contributes to the tumorigenesis of bladder urothelial carcinoma by regulating MLK3/NF-kappaB pathway. Am J Transl Res 7(12):2786–2794
Szarvas T et al (2011) Matrix metalloproteinases and their clinical relevance in urinary bladder cancer. Nat Rev Urol 8(5):241–254
Wang Y et al (2020) GBX2, as a tumor promoter in lung adenocarcinoma, enhances cells viability, invasion and migration by regulating the AKT/ERK signaling pathway. J Gene Med 22(2):e3147
Xiao Y et al (2018) Integrin alpha5 down-regulation by miR-205 suppresses triple negative breast cancer stemness and metastasis by inhibiting the Src/Vav2/Rac1 pathway. Cancer Lett 433:199–209
Xu X et al (2016) c-Met and CREB1 are involved in miR-433-mediated inhibition of the epithelial-mesenchymal transition in bladder cancer by regulating Akt/GSK-3beta/Snail signaling. Cell Death Dis 7:e2088
Funding
This study was supported by National Natural Science Foundation of China (No. 81802561) and Natural Science Foundation of Hunan Province, China (No. 2019JJ50977).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 16 kb)

Fig. S1
Images for Wound healing and Transwell assays performed on bladder cancer cells transfected with sh-GBX2 or GBX2 oe. (PNG 7625 kb)

Fig. S2
Images for Wound healing and Transwell assays performed on bladder cancer cells transfected with sh-ITGA5 or ITGA5 oe. (PNG 6612 kb)

Fig. S3
Images for Transwell assays performed on bladder cancer cells co-transfected with sh-GBX2 and ITGA5 oe. (PNG 2903 kb)
Rights and permissions
About this article
Cite this article
Xiong, Y., Song, X., Kudusi et al. Oncogenic GBX2 promotes the malignant behaviors of bladder cancer cells by binding to the ITGA5 promoter and activating its transcription. Funct Integr Genomics 22, 937–950 (2022). https://doi.org/10.1007/s10142-022-00870-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-022-00870-8