Abstract
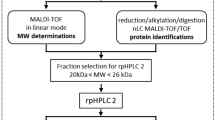
Pheromones are considered to play an important role in broadcast spawning in aquatic animals, facilitating synchronous release of gametes. In oysters, the sperm has been implicated as a carrier for the spawn-inducing pheromone (SIP). In hatchery conditions, male pearl oysters (Pinctata maxima) can be stimulated to spawn through a variety of approaches (e.g. rapid temperature change), while females can only be induced to spawn through exposure to conspecific sperm, thus limiting development of targeted pairing, required for genetic research and management. The capacity for commercial production and improvement of genetic lines of pearl oysters could be greatly improved with access to a SIP. In this study, we prepared and sequenced crude and semi-purified P. maxima sperm extracts that were used in bioassays to localise the female SIP. We report that the P. maxima SIP is proteinaceous and extrinsically associated with the sperm membrane. Bioactivity from pooled RP-HPLC fractions, but not individual fractions, suggests that the SIP is multi-component. We conclude that crude sperm preparations, as described in this study, can be used as a sperm-free inducer of female P. maxima spawning, which enables for a more efficient approach to genetic breeding.


Similar content being viewed by others
References
Agelopoulou B, Cary PD, Pataryas T, Aleporou-Marinou V, Crane-Robinson C (2004) The sperm-specific proteins of the edible oyster (European flat oyster (Ostrea edulis)) are products of proteolytic processing. Biochim Biophys Acta 1676(1):12–22
Appelt CW, Sorensen PW (2007) Female goldfish signal spawning readiness by altering when and where they release a urinary pheromone. Anim Behav 74(5):1329–1338
Boo KS (1998) Variation in sex pheromone composition of a few selected lepidopteran species. J Asia Pac Entomol 1(1):17–23
Chung-Davidson Y-W, Huertas M, Li W 2010 A review of research in fish pheromones. Chem Commun Crustac 467–482. https://doi.org/10.1007/978-0-387-77101-4_24
Cummins SF, Schein CH, Xu Y, Braun W, Nagle GT (2005) Molluscan attractins, a family of water-borne protein pheromones with interspecific attractiveness. Peptides 26(1):121–129
Cummins SF, Boal JG, Buresch KC, Kuanpradit C, Sobhon P, Holm JB, Degnan BM, Nagle GT, Hanlon RT (2011) Extreme aggression in male squid induced by a β-MSP-like pheromone. Curr Biol 21(4):322–327
Eom J, Jung YR, Park D (2009) F-series prostaglandin function as sex pheromones in the Korean salamander, Hynobius leechii. Comp Biochem Physiol A Mol Integr Physiol 154(1):61–69
Fine J, Sorenson PW (2008) Isolation and biological activity of the multi-component sea lamprey migratory pheromone. J Chem Ecol 34(10):1259–1267
Galtsoff P (1938) Physiology of reproduction of Ostrea virginica II. Stimulation of spawning in the female oyster. Biol Bull 75(2):286–307
Jonáková V, Manásková P, Tichá M (2007) Separation, characterization and identification of boar seminal plasma proteins. J Chromatogr B 849(1–2):307–314
Kikuyama S, Toyoda F, Ohmiya Y, Matsuda K, Tanaka S, Hayashi H (1995) Sodefrin: a female-attracting peptide pheromone in newt cloacal glands. Science 267(5204):1643–1645
Kuanpradit C, Stewart MJ, York PS, Degnan BM, Sobhon P, Hanna PJ, Chavadej J, Cummins SF (2012) Characterization of mucus-associated proteins from abalone (Haliotis) – candidates for chemical signaling. FEBS J 279:437–450
Lessios HA (2011) Speciation genes in free-spawning marine invertebrates. Integr Comp Biol 51(3):456–465
Li W, Scott AP, Siefkes MJ, Yan H, Liu Q, Yun S-S, Gage DA (2002) Bile acids secreted by male sea lamprey that acts as a sex pheromone. Science 296(5565):138–141
Mamangkey NGF, Southgate PC (2009) Regeneration of excised mantle tissue by the silver-lip pearl oyster, Pinctada maxima (Jameson). Fish Shellfish Immunol 27(2):164–174
Minaur J (1969) Experiments on the artificial rearing of the larvae of the Pinctada maxima (Jameson) (Lamellibranchia). Aust J Mar Freshwat Res 20:175–187
Painter SD, Clough B, Black S, Nagle GT (2003) Behavioral characterization of attractin, a water-borne peptide pheromone in the genus Aplysia. Biol Bull 25(1):16–25
Queensland South Sea Pearls (2011) Artificial spat culture in Australia. http://www.qssp.com.au/spat_culture%20.htm. Viewed 10 March 2011
Reddy GVP, Guerrero A (2010) New pheromones and insect control strategies. In: Gerald L (ed) Vitamins & hormones, vol. 83. Academic Press, Oxford, pp. 493–519
Rice P, Ray SM, Painter SD, Nagle GT (2002) Intrinsic membrane protein in oyster sperm stimulates spawning behaviours in Crassostrea virginica: implications for aquaculture. J Shellfish Res 21(2):715–718
Rose RA (1990) A manual for the artificial propagation of the silverlip or goldlip pearl oyster, Pinctada maxima, (Jameson) from Western Australia’ Fishries Department, Western Australian Marine Research Laboratories, P.O. Box 20, North Beach, WA 6020, Australia
Snell TW, Nacionales MA (1990) Sex pheromone communication in Brachionus plicatilis (rotifera). Comp Biochem Physiol A Physiol 97(2):211–216
Solis N, Larsen MR, Cordwell SJ (2010) Improved accuracy of cell surface shaving proteomics in Staphylococcus aureus using a false-positive control. Proteomics 10:2037–2049
Stacey NE, Sorensen PW, Van Der Kraak GJ, Dulka JG (1989) Direct evidence that 17[alpha],20[beta]-dihydroxy-4-pregnen-3-one functions as a goldfish primer pheromone: Preovulatory release is closely associated with male endocrine responses. Gen Comp Endocrinol 75(1):62–70
Suzuki N (1995) Structure, function and biosynthesis of sperm-activating peptides and fucose sulfate glycoconjugate in the extracellular coat of sea urchin eggs. Zool Sci 12(1):13–27
Tanaka Y, Kumeta M (1981) Successful artificial breeding of the silver lip pearl oyster, Pinctada maxima (Jameson). Bull Natl Res Inst Aquac (Jpn) 2:21–28
Taverna F, Negri A, Piccinini R, Zecconi A, Nonnis S, Ronchi S, Tedeschi G (2007) Characterization of cell wall associated proteins of a Staphylococcus aureus isolated from bovine mastitis case by a proteomic approach. Vet Microbiol 119:240–247
Tillman JA, Seybold SJ, Jurenka RA, Blomquist GJ (1999) Insect pheromones—an overview of biosynthesis and endocrine regulation. Insect Biochem Mol Biol 29(6):481–514
Tranter DJ (1958) Reproduction in Australian pearl oyster (Lamellibranchia). II. Pinctada albina (Lamarck): Gametogenesis. Aust J Mar Freshwat Res 9:144–158
Vertomenn A, Panis B, Swennen R, Carpentier SC 2011 Challenges and solutions for the identification of membrane proteins in nonmodel plants. J Proteomics
Wang T, Zhao M, Rotgans BA, Strong A, Liang D, Ni G, Limpanont Y, Ramasoota P, Mcmanus DP, Cummins SF (2016) Proteomic Analysis of the Schistosoma mansoni Miracidium. PLoS One 11:e0147247
Acknowledgements
We would like to thank Paspaley Pearling Company for providing the pearl oysters used in this study, the Darwin Aquaculture Centre, Northern Territory Department of Primary Industry and Resources for the use of their facilities, and the University of the Sunshine Coast for use of the Genecology labs.
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Taylor, A., Mills, D., Wang, T. et al. A Sperm Spawn-Inducing Pheromone in the Silver Lip Pearl Oyster (Pinctada maxima). Mar Biotechnol 20, 531–541 (2018). https://doi.org/10.1007/s10126-018-9824-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-018-9824-6